Metagenomics virus detection
The TELEVIR bioinformatics component of INSaFLU is a modular pipeline for the identification of viral sequences in metagenomic data (both Illumina and ONT data).
It is composed of these main steps (detailed in https://insaflu.readthedocs.io/en/latest/bioinformatics_pipeline.html#metagenomics-virus-detection):
Read quality analysis and improvement [optional].
Extra filtering [optional].
Viral Enrichment [optional].
Host Depletion [optional].
De novo Assembly of the reads [optional].
- Identification of the viral sequences.
Using reads.
Using contigs (if assembled).
Using several reference databases.
Selection of viral TAXID and representative genome sequences for confirmatory re-mapping.
Remapping of the viral sequences against selected reference genomes.
Reporting.
The following table provides an overview on all TELEVIR moules and outputs:
TELEVIR_current_modules_and_outputs_2023-10-20.xlsx
Below, you can find instructions on how to create a TELEVIR project, run samples and visualize/intrepret the results.
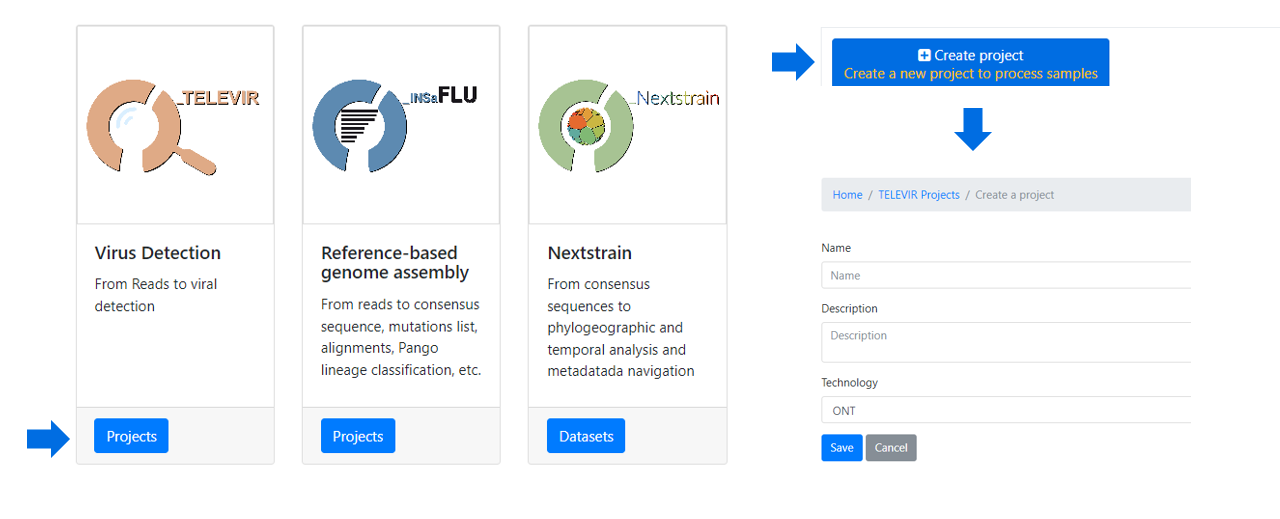
TELEVIR Projects - How to create and run a metagenomics virus detection project
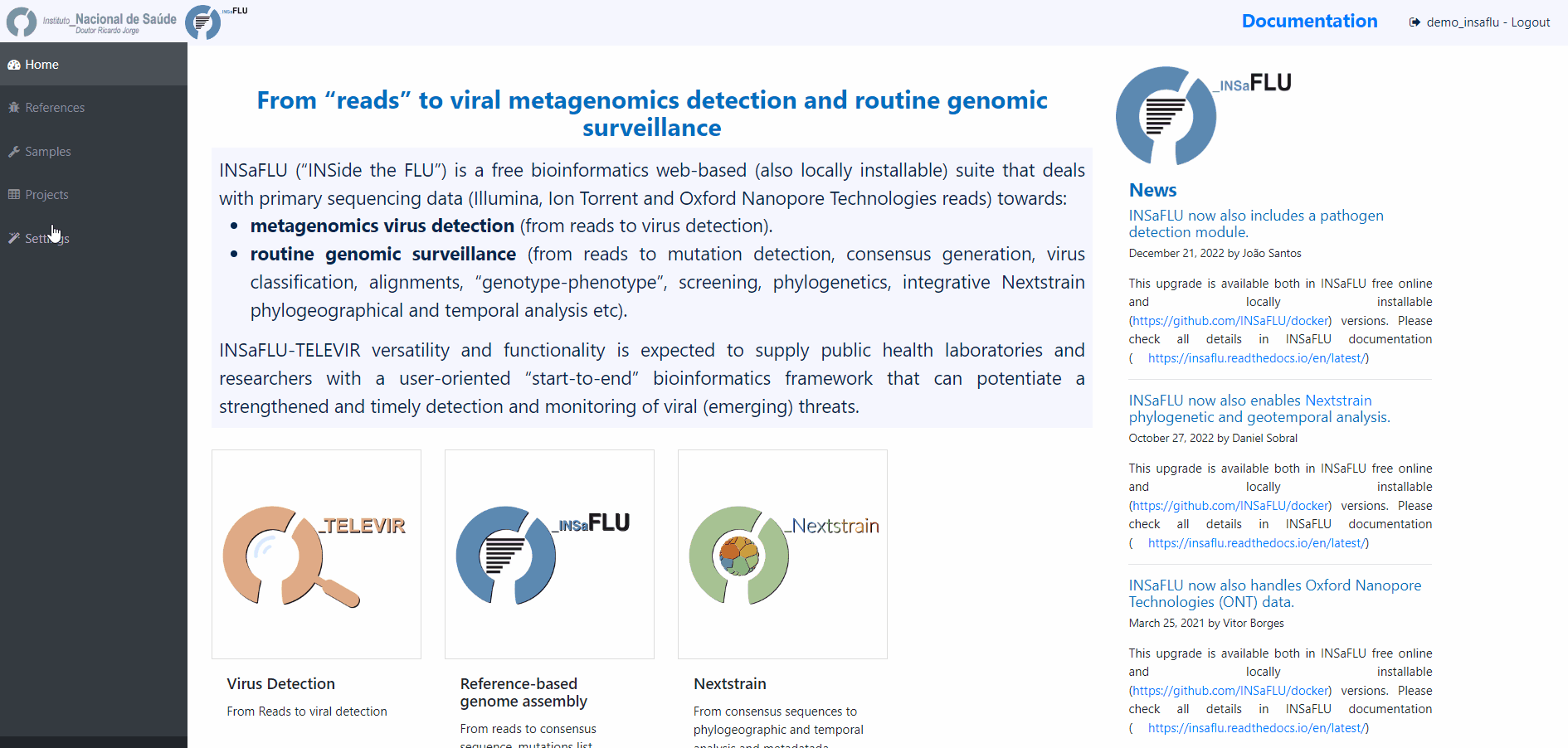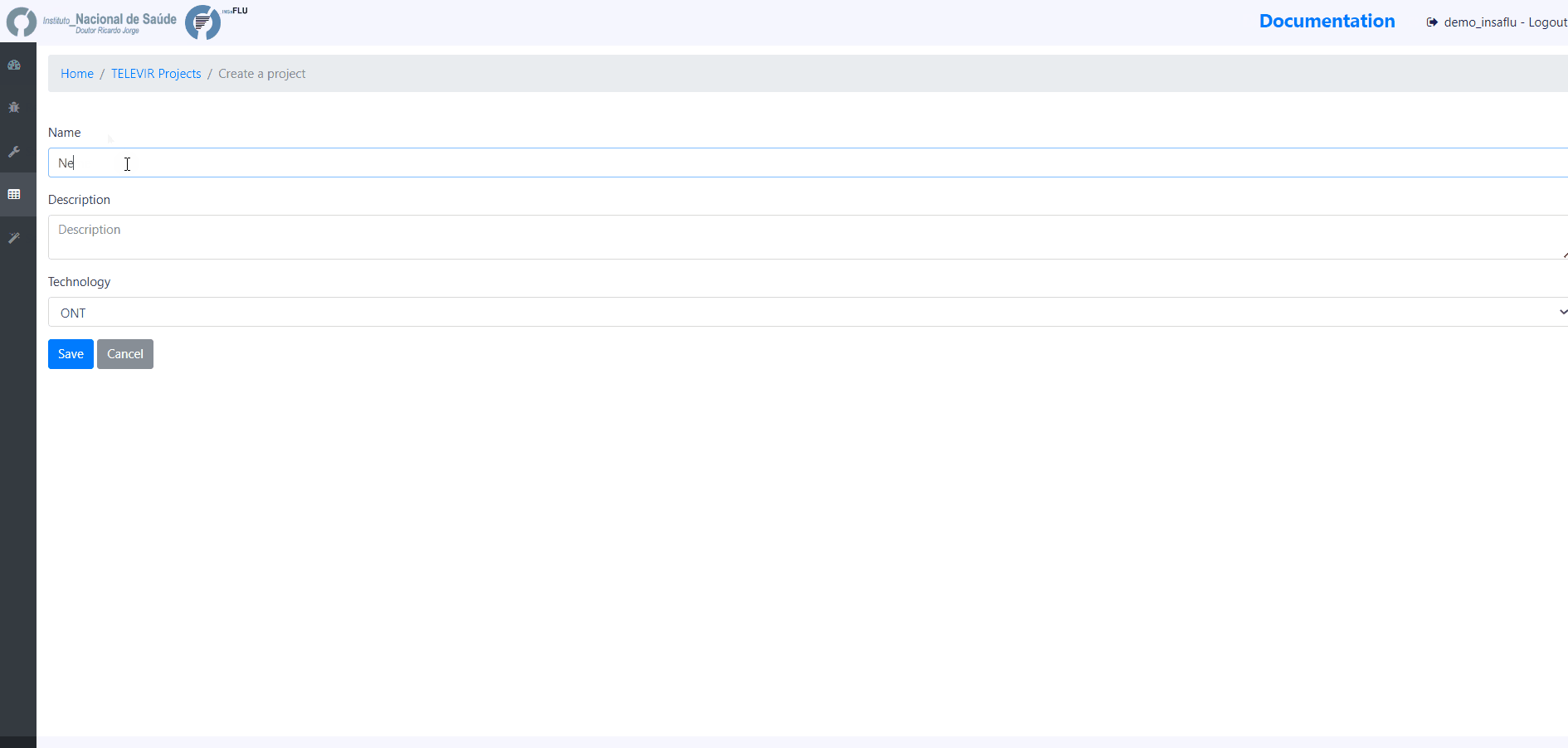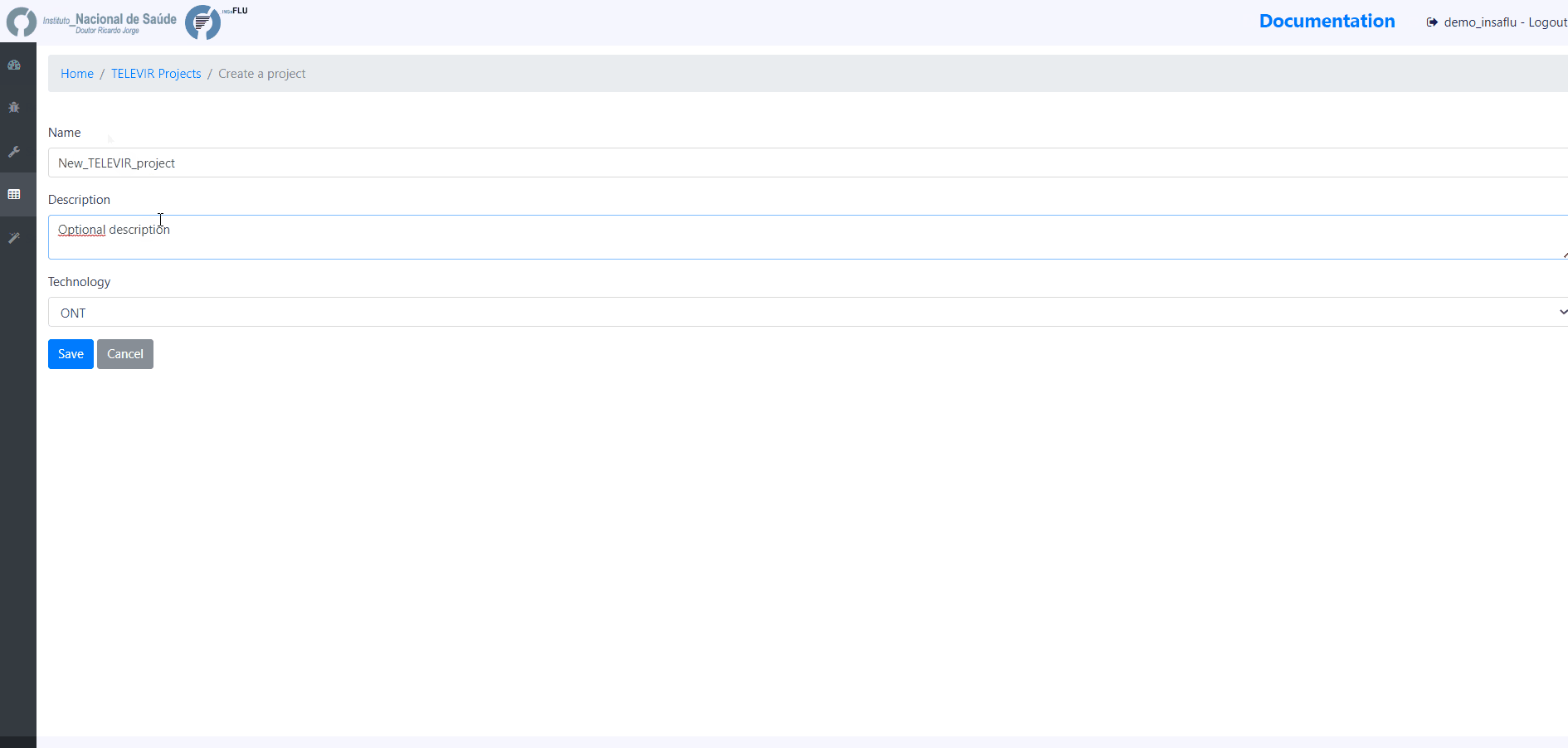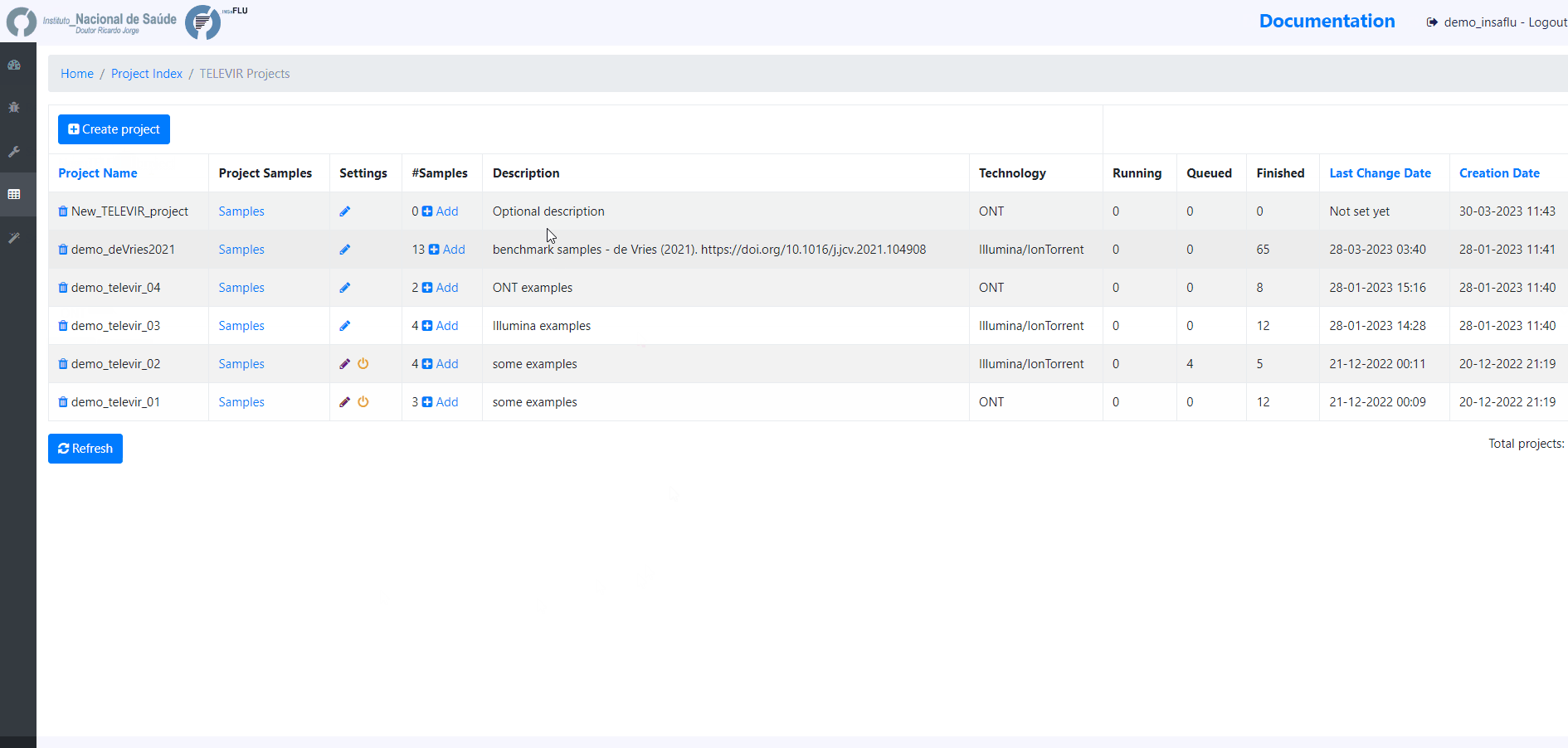
Within the TELEVIR Projects menu:
2. Add a Name and Description, Choose the Sequencing technology and Save
After creating a project, and before adding/running samples, please:.
3. Select the Workflow and Software to be run
As there is no “one-size-fits-all” bioinformatics pipeline that can detect all viruses, the TELEVIR module was designed to allow users to easily run complex workflows simultaneously (covering several combinations of classification algorithms, databases and parameters, etc).
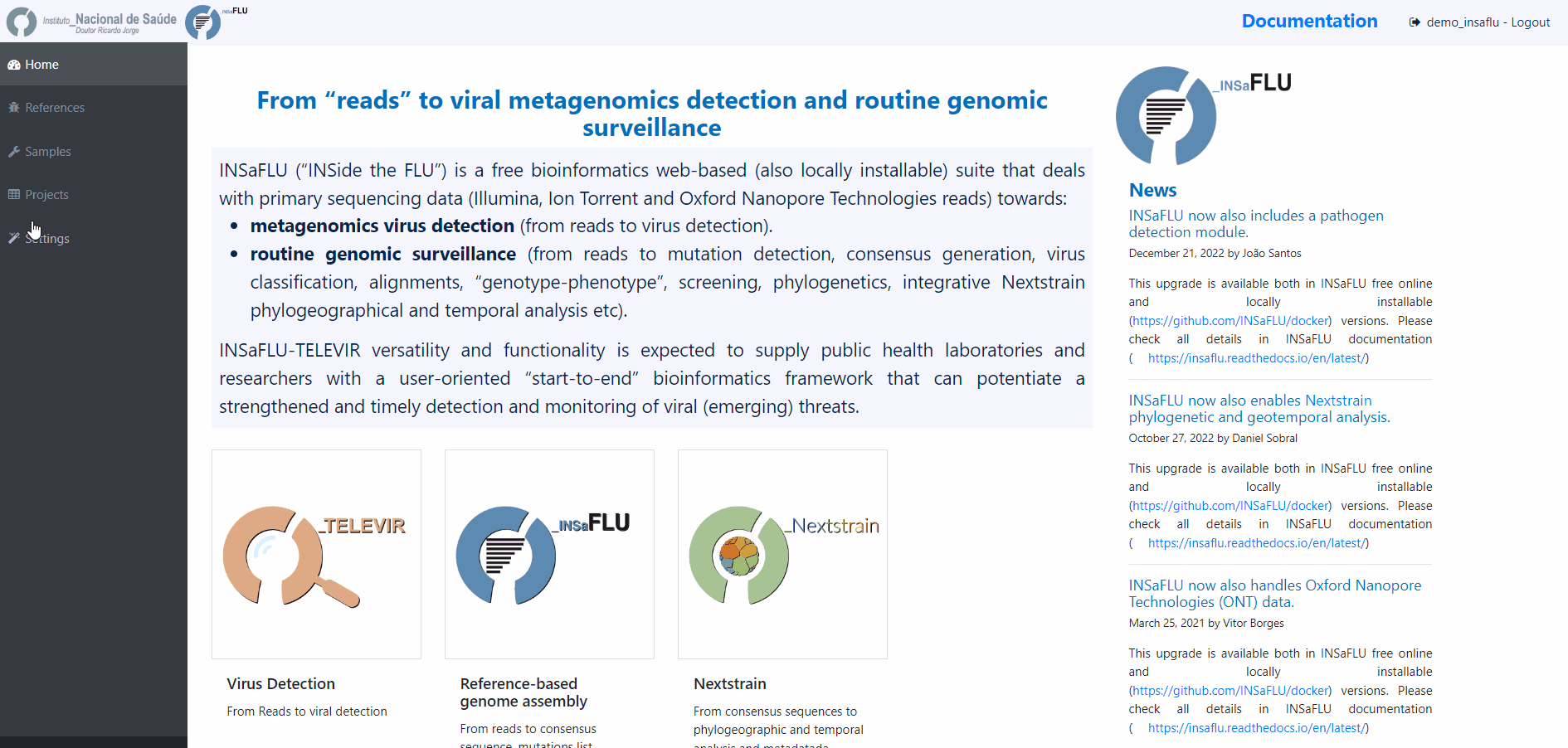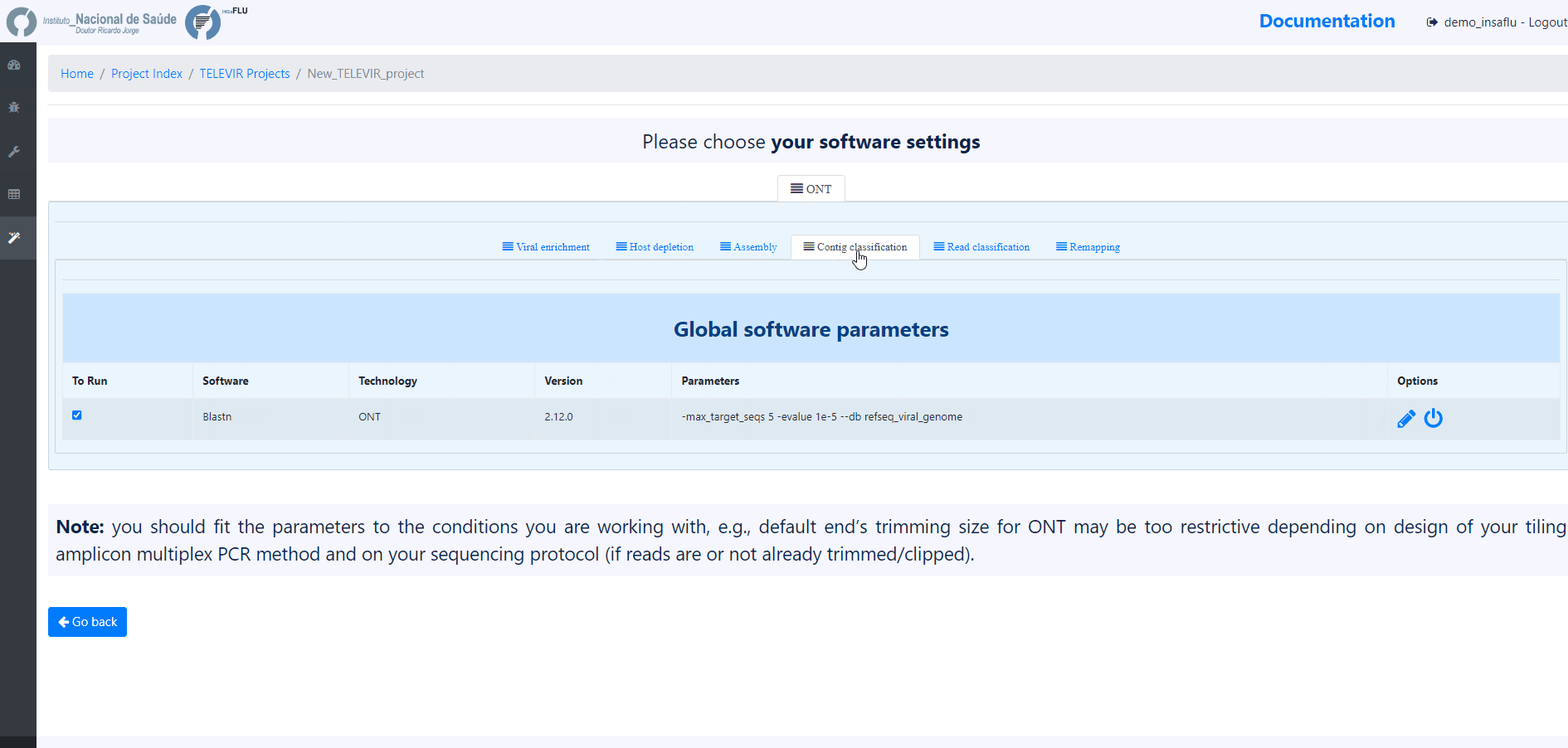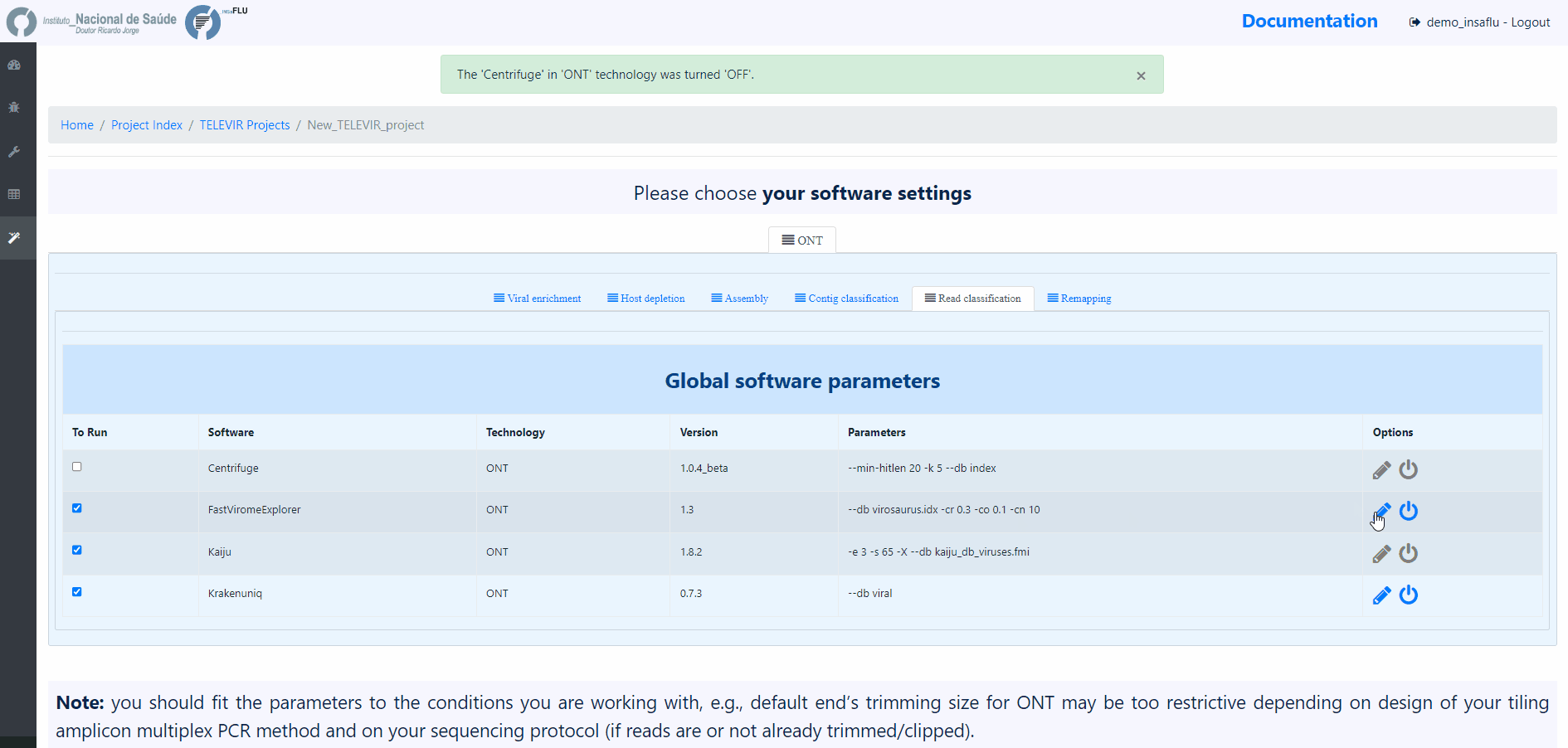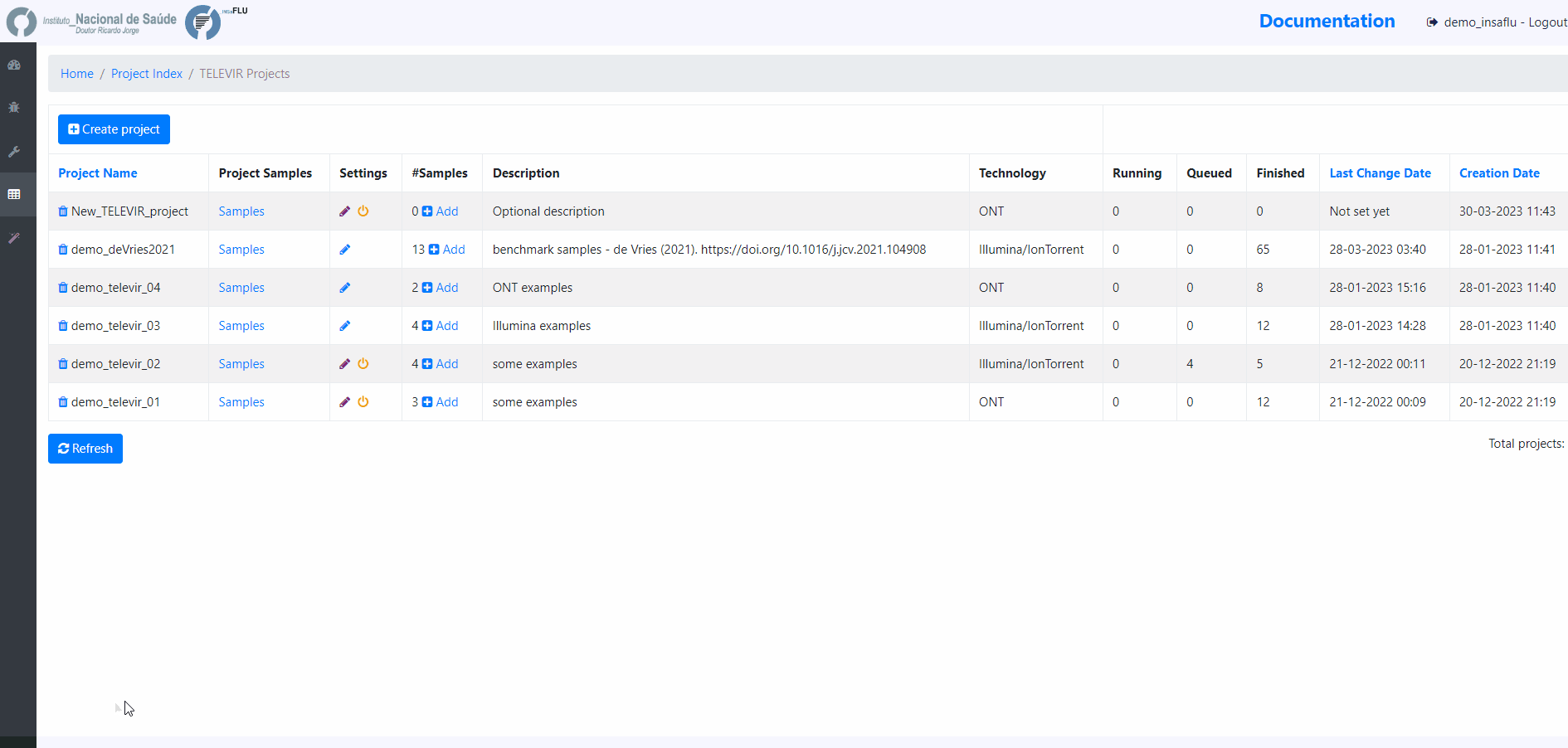
Workflows and parameters can be changed at the global level, through the settings menu, or specifically for an existing project by clicking the “Magic Wand” icon on an existing project’s listing in the TELEVIR Projects page. Project settings will apply only to deployments within that project. Conversely, Global settings apply only to projects that have not had their settings changed.
Note
The TELEVIR Settings page controls the bioinformatics workflows to be applied. Inside, software are organized by technology and pipeline. Controlling workflows is done by selecting/deselecting which software are to run at each step of the pipeline, their parameters and/or databases when permitted. Specific steps can be turned off by deselecting all software available for that step
The default workflows are “well-performing” workflows (selected after multiple testing and benchmarking) that together can potentiate the detection of clinical relevant viruses.
NOTE: Some pipeline steps cannot be turned off (e.g. Remapping). Other cases are context dependent: Assembly cannot be turned OFF if Contig Classification is turned ON; at least one classification step must be turned ON (Contig Classification may not be turned OFF if Read Classification is already OFF, and vice-versa).
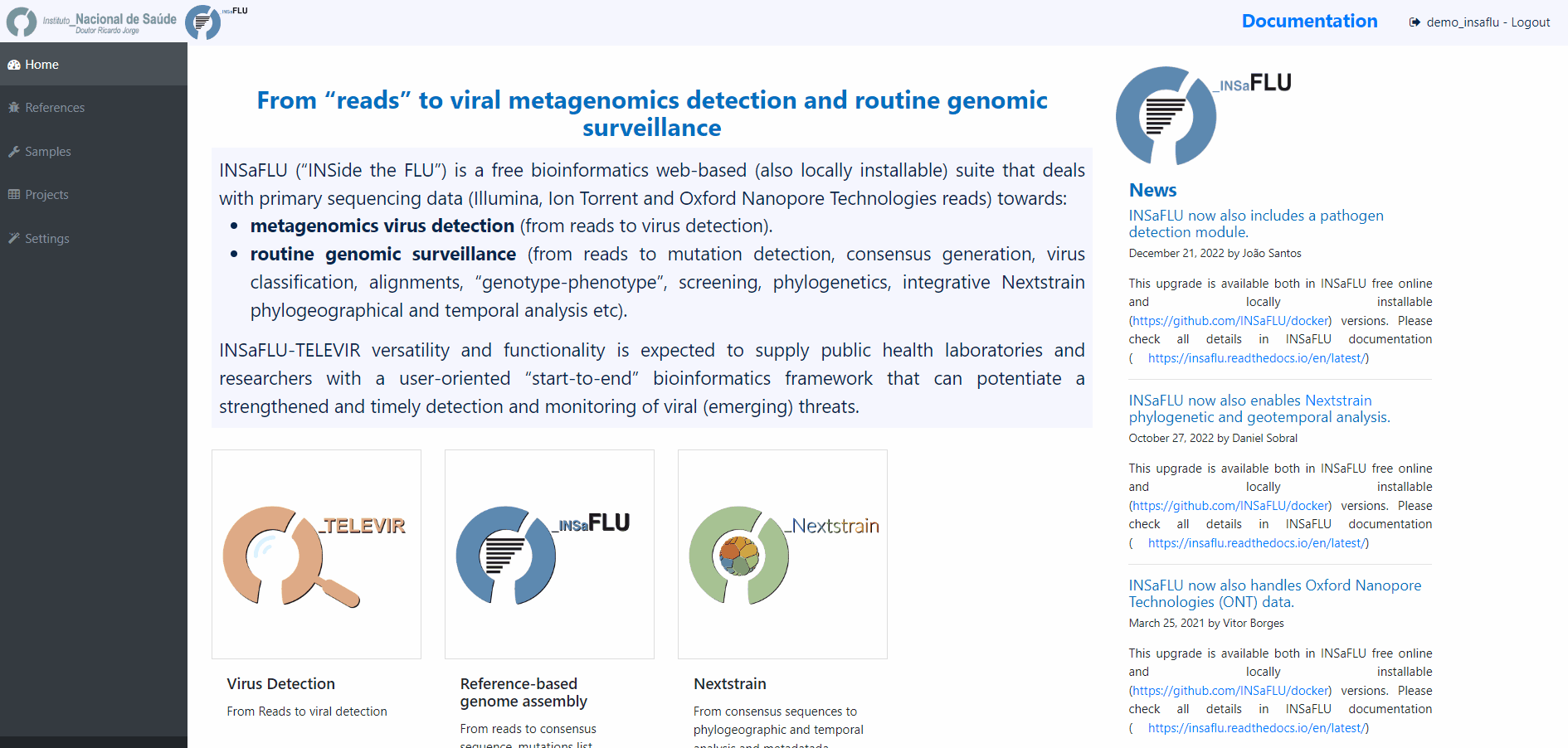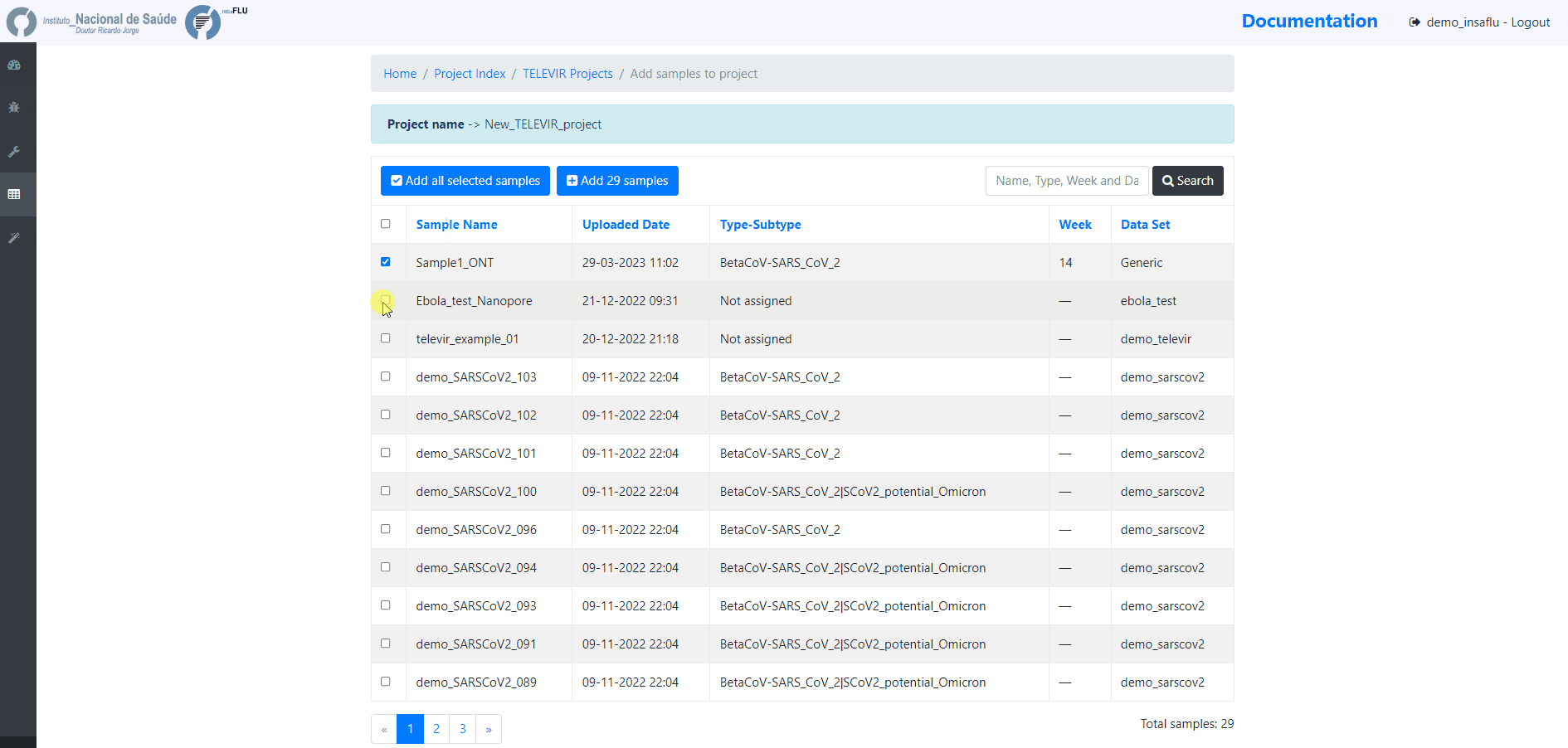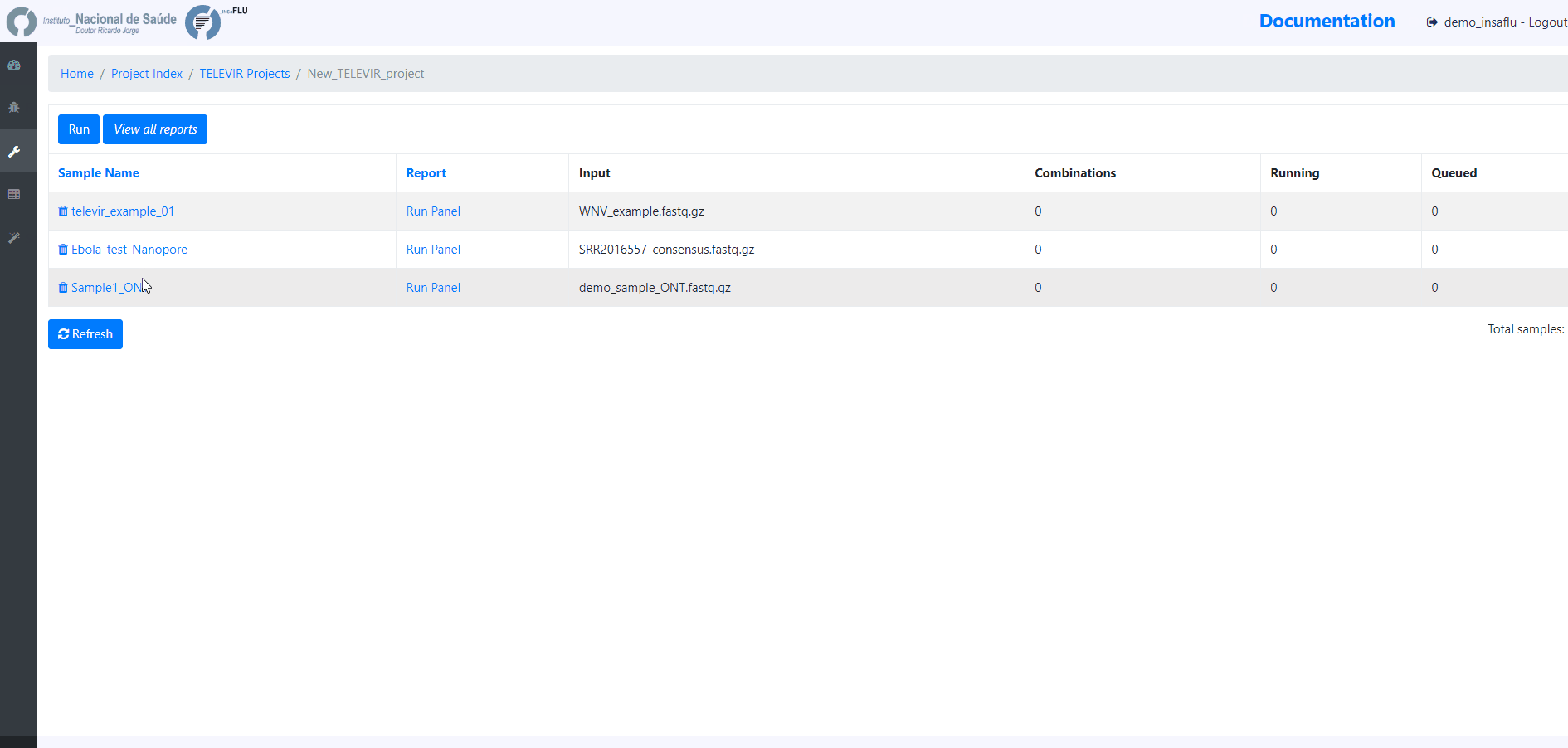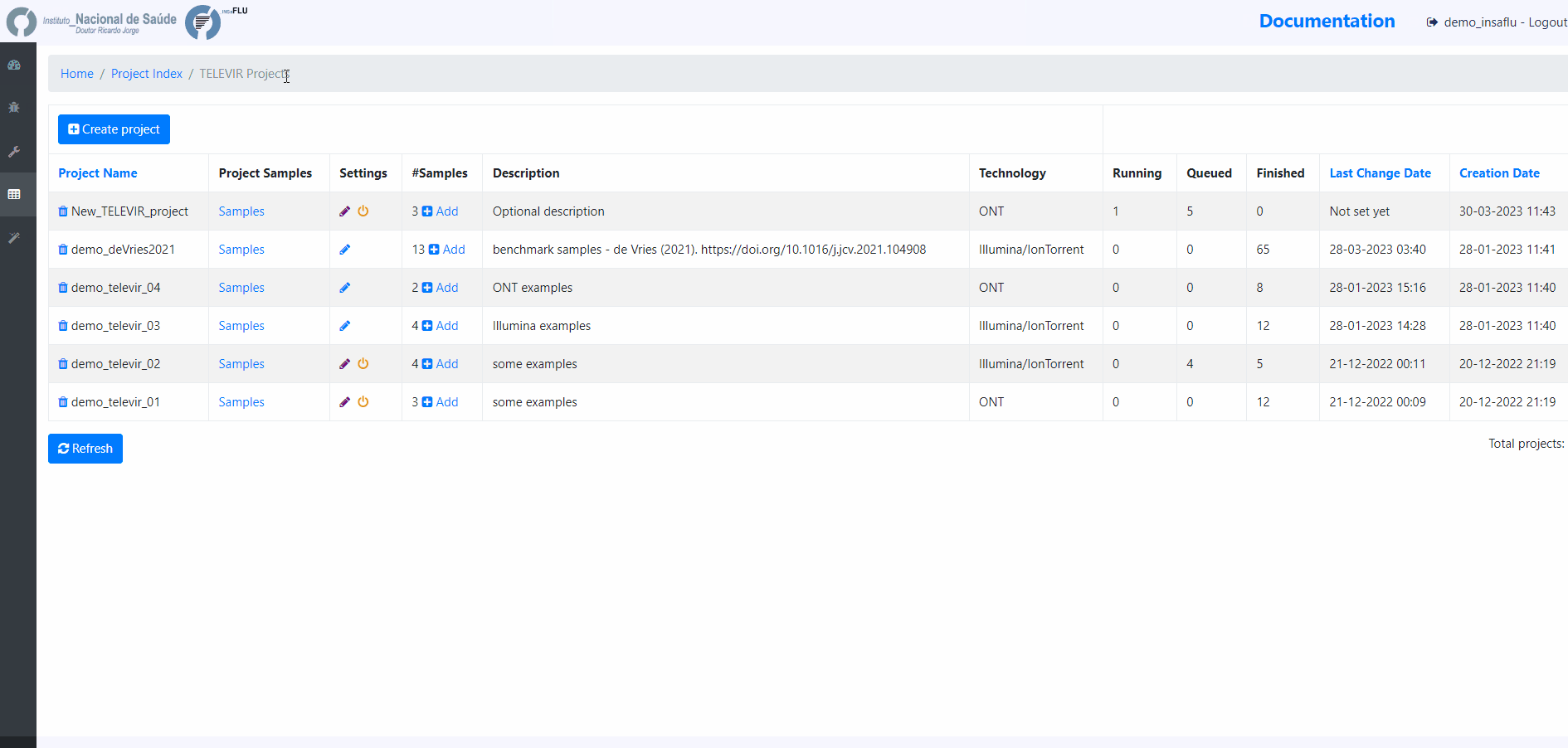
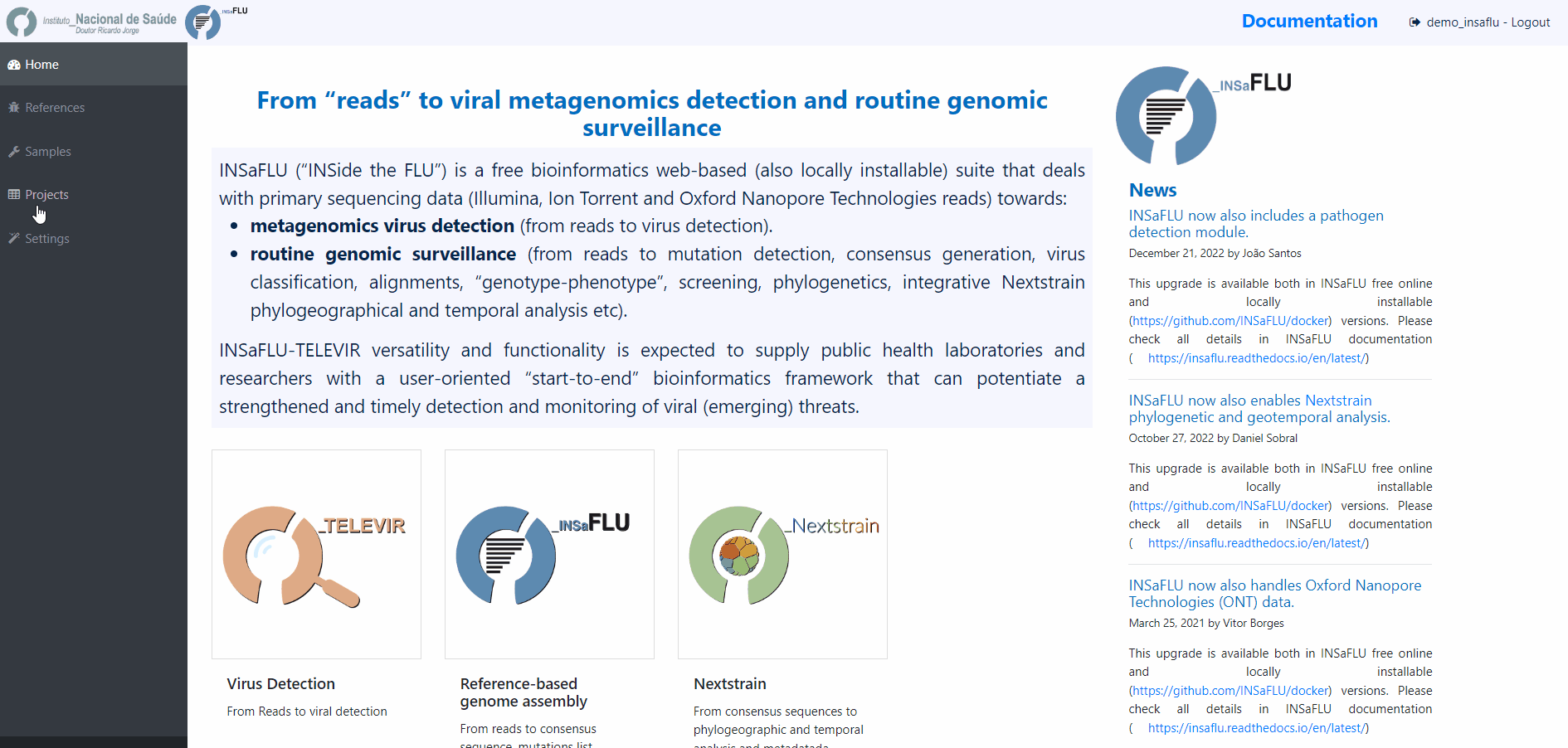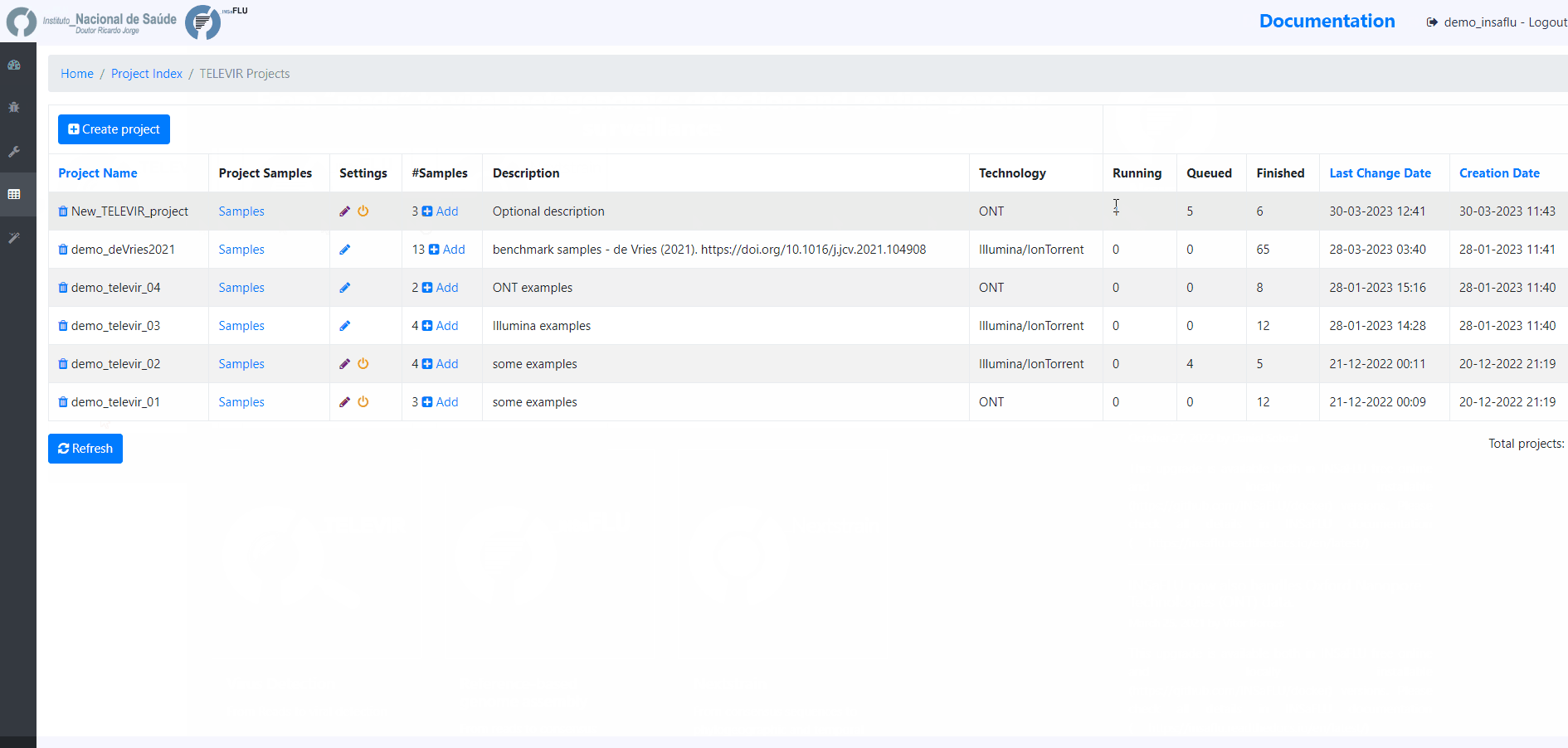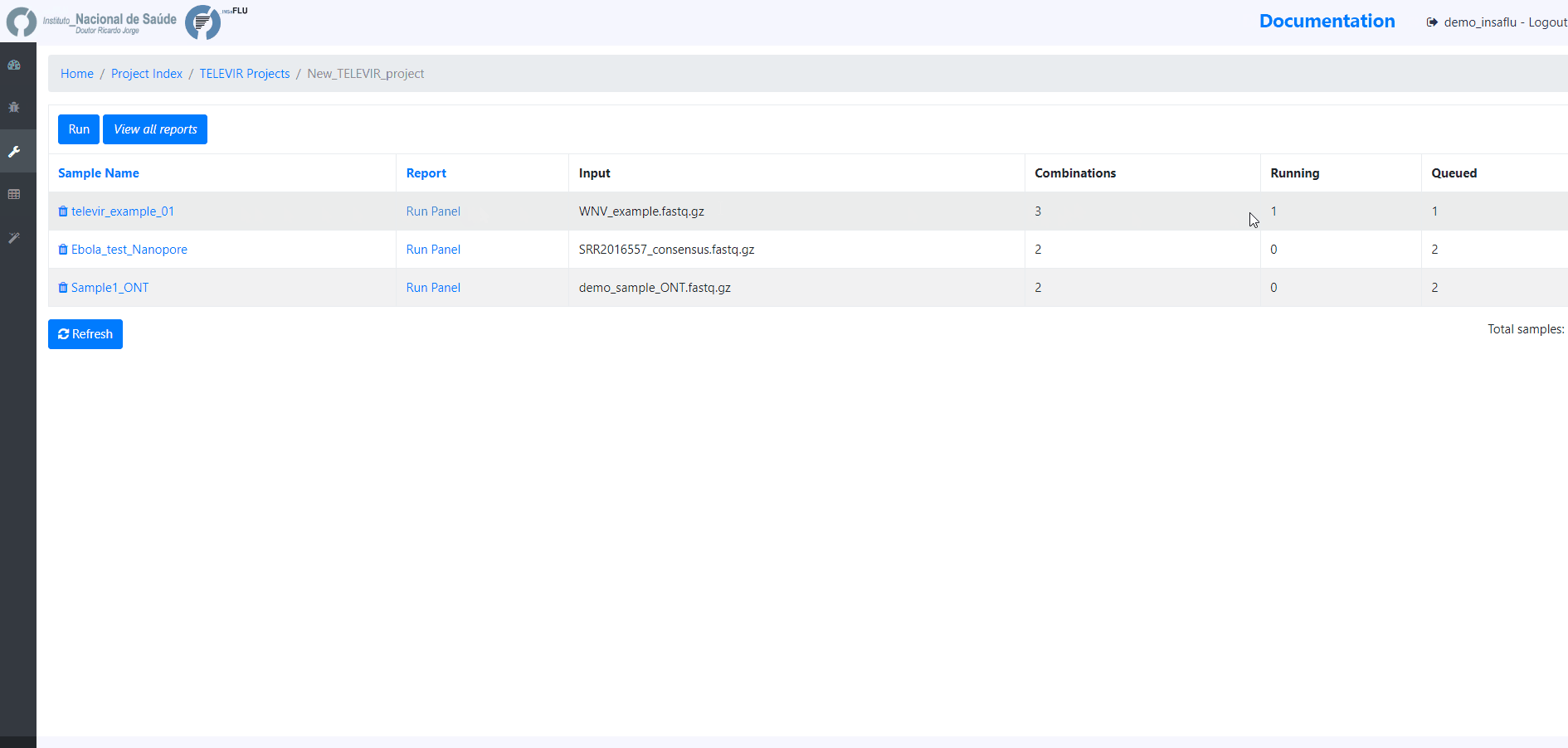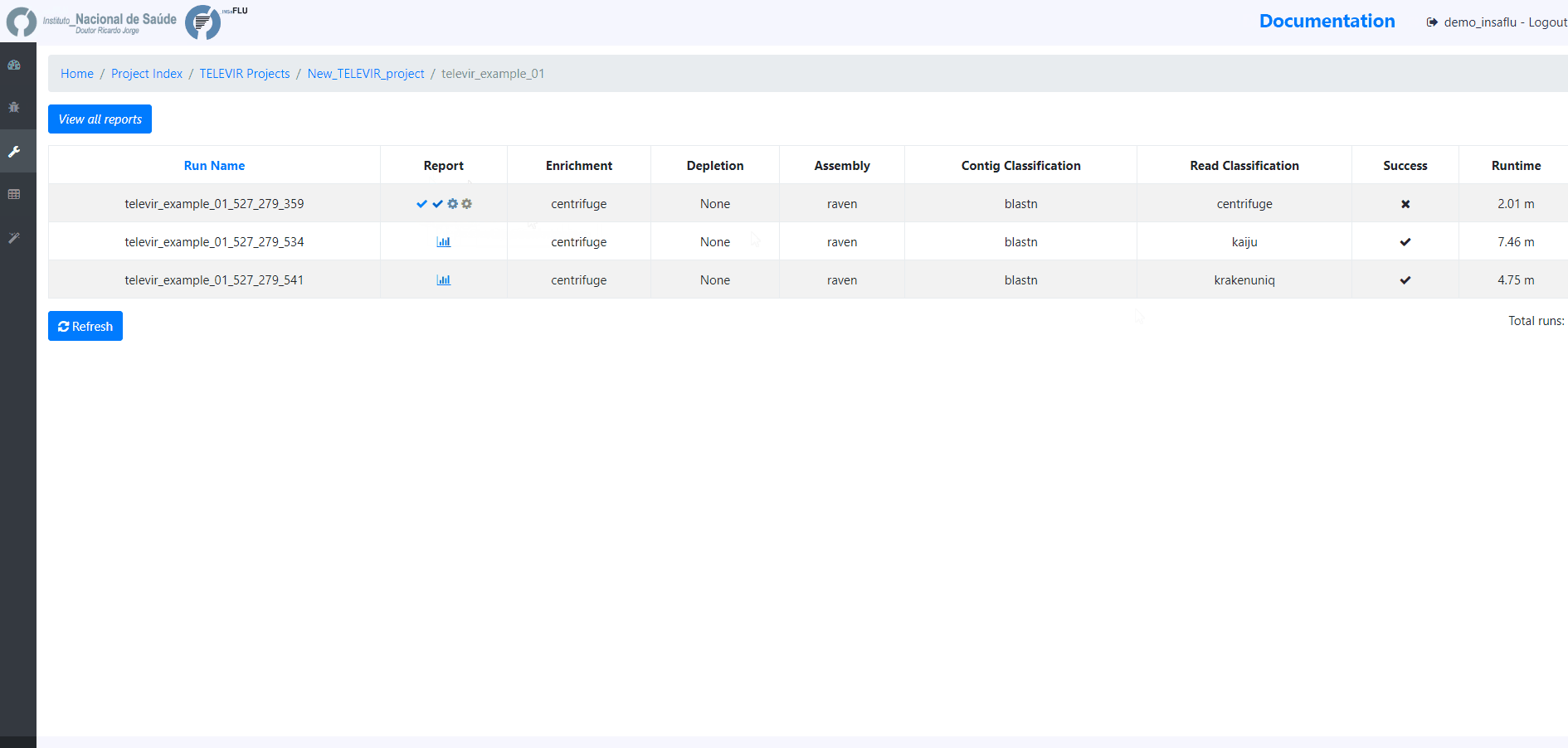
4. Add samples to the TELEVIR project and click in Run
Before running the added samples, please make sure that you have previously selected the bioinformatics workflows (“runs”) to be applied to every sample added to the project using the “Magic wand” (see previous step)
Note
At any time, users can change parameters to run new workflows. Results will be integrated to the Combined Report cumulatively.
At this time, users may start monitoring the Project progress by checking the runs (i.e., combinations of workflows) “Queued” or “Running”. Workflows are applied to every sample assigned to this project.
By clicking in Run panel, users can get an overview of the workflows run.
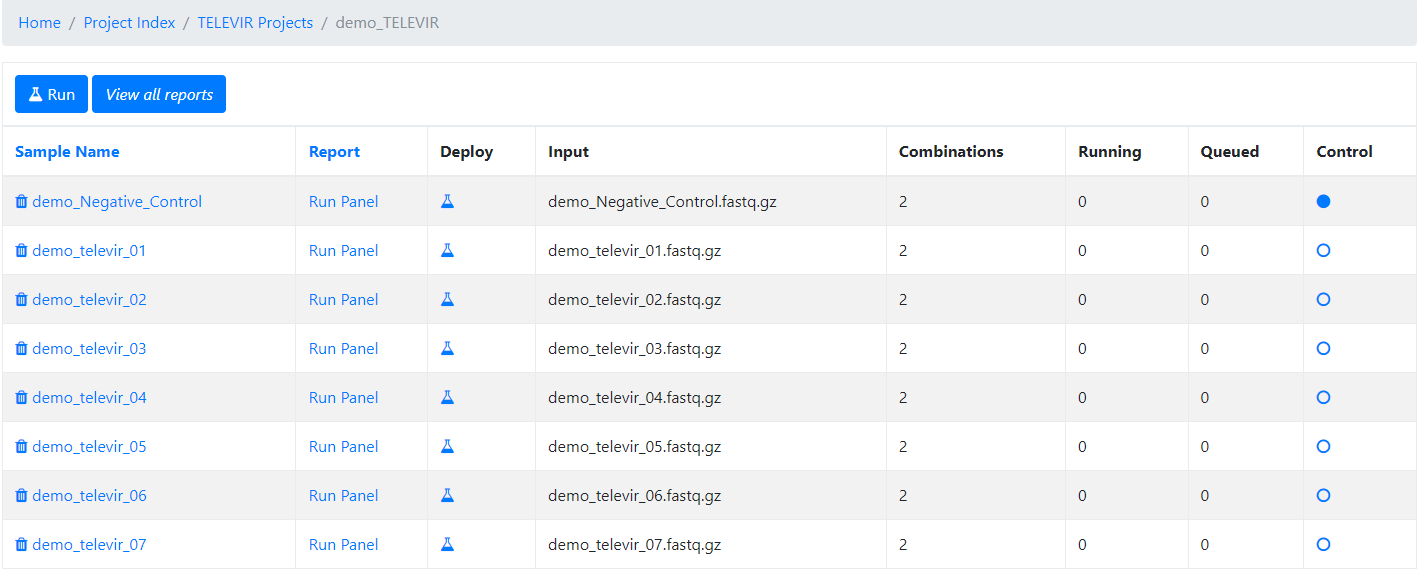
5. Select a Control to the TELEVIR project
The inclusion of negative controls (e.g. pathogen-negative samples, library preparation buffers, etc) during metagenomic sequencing in clinical virology is highly recommended to identify sources of potential contamination and detect false positive hits. In addition, the inclusion of positive controls (e.g., samples spiked with RNA or DNA viruses that do not infect humans) is also commonly performed to control for the success of nucleic acids extraction, preparation and sequencing.
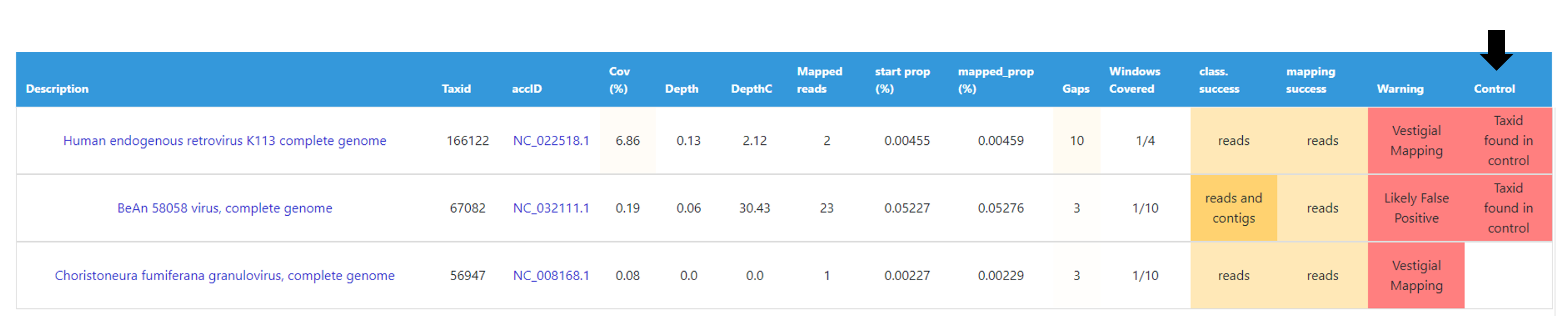
In TELEVIR projects, user can select “control” sample(s) at any time (before and after data analysis). Viral TAXID detected in the Main report of the user-selected “control” sample(s) will be flagged in the reports of samples in the same project as “Taxid found in control” in a new “Control” column. This functionality is designed to facilitate the background subtraction of viral hits also found in controls. Multiple controls are possible.
TELEVIR - Output Visualization and Download
The INSaFLU-TELEVIR bioinformatics pipeline for metagenomics virus diagnostic generates multiple outputs, reflecting the multiple steps of the pipeline (detailed here: https://insaflu.readthedocs.io/en/latest/bioinformatics_pipeline.html#metagenomics-virus-detection). The main report lists the top viral hits, each accompanied by several robust and diagnostic-oriented metrics, statistics and visualizations, provided as (interactive) tables (intermediate and final reports), graphs (e.g., coverage plots, Integrative Genomics Viewer visualization, Assembly to reference dotplot) and multiple downloadable output files (e.g., list of the software parameters, reads/contigs classification reports, mapped reads/contigs identified per each virus; reference sequences, etc)
TELEVIR reports are generated per Workflow, per Sample (combining non-redundant hits detected across workflows) and per Project (combining several samples), with a decreasing level of detail.
Workflow Reports are organized in dynamic ‘expand-and-collapse’ panels:
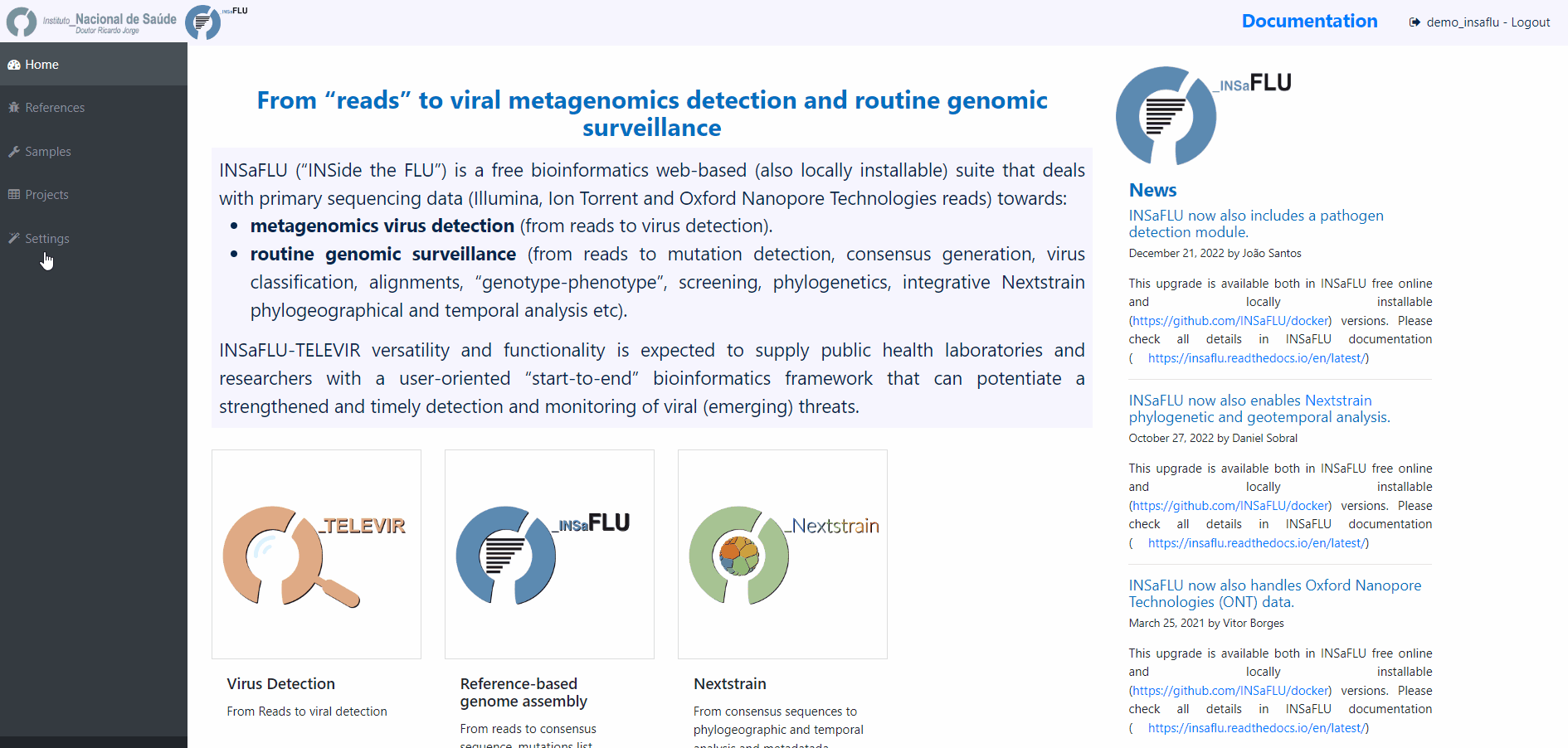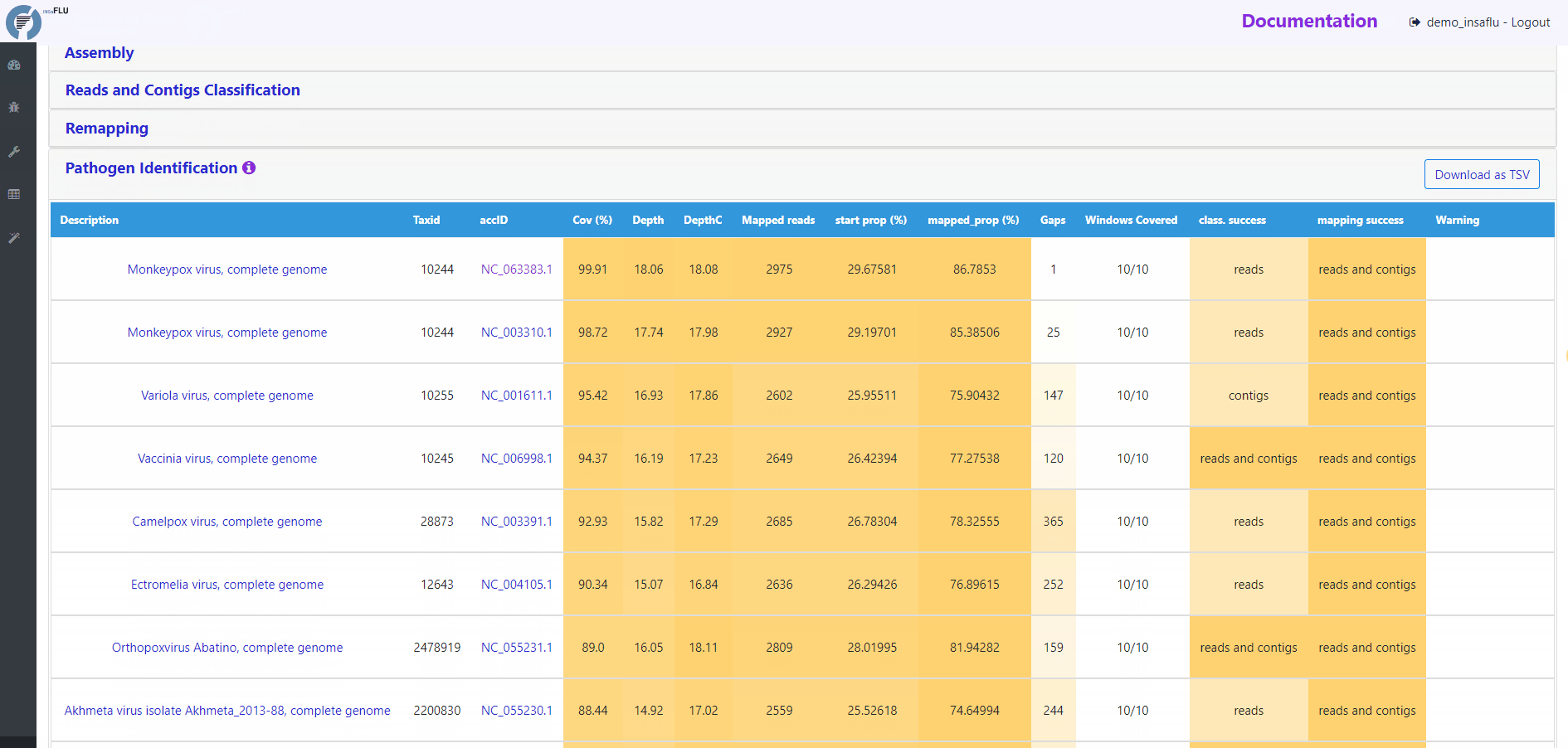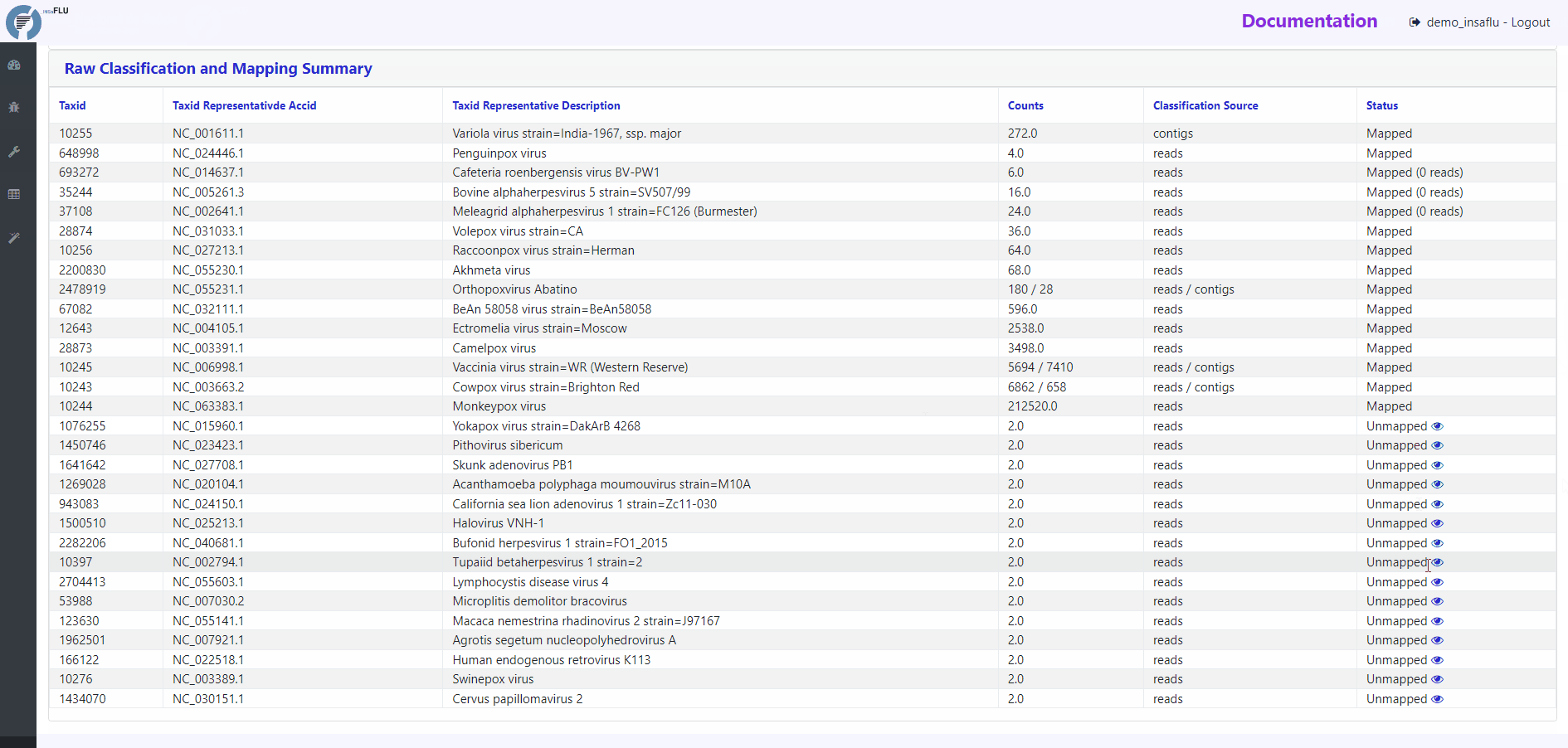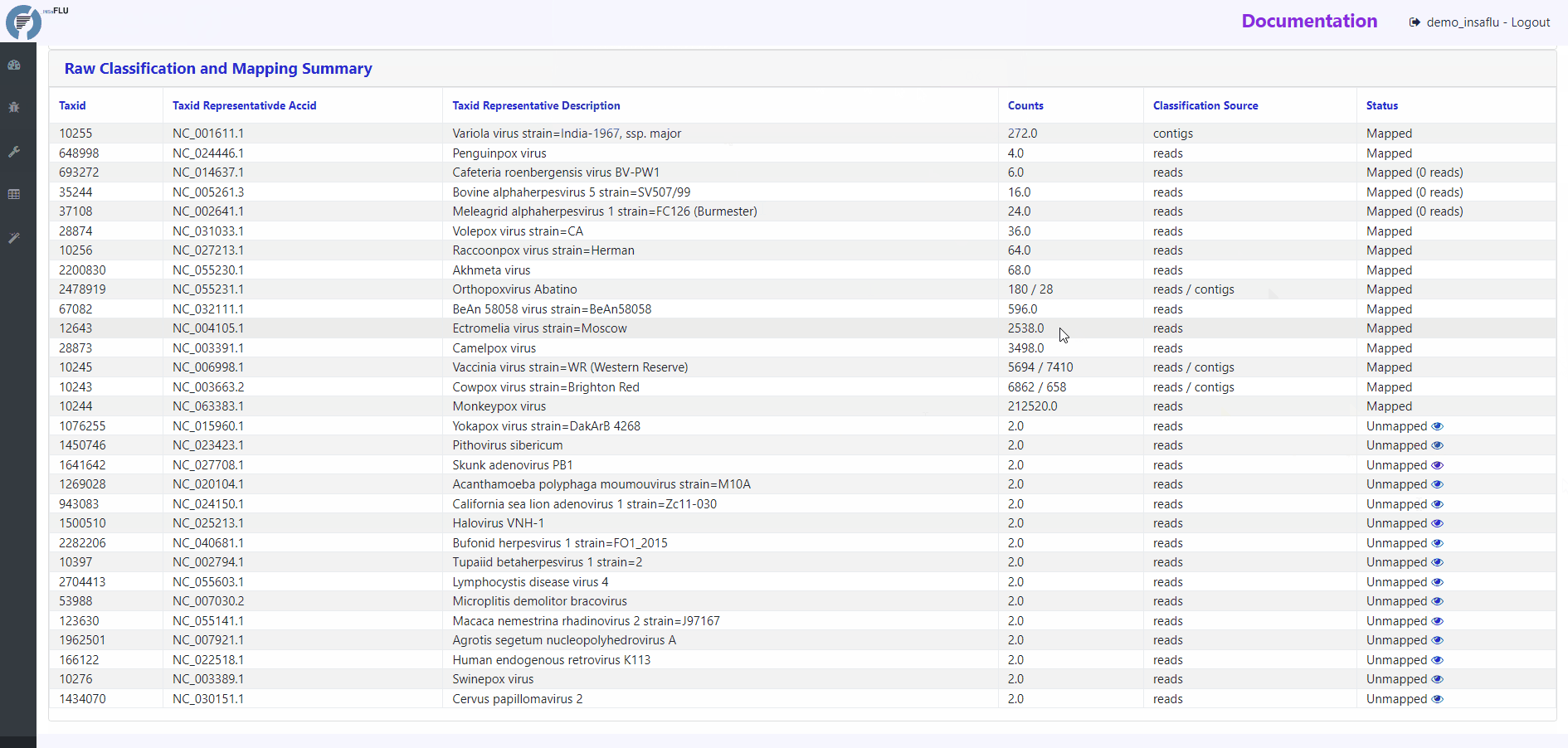
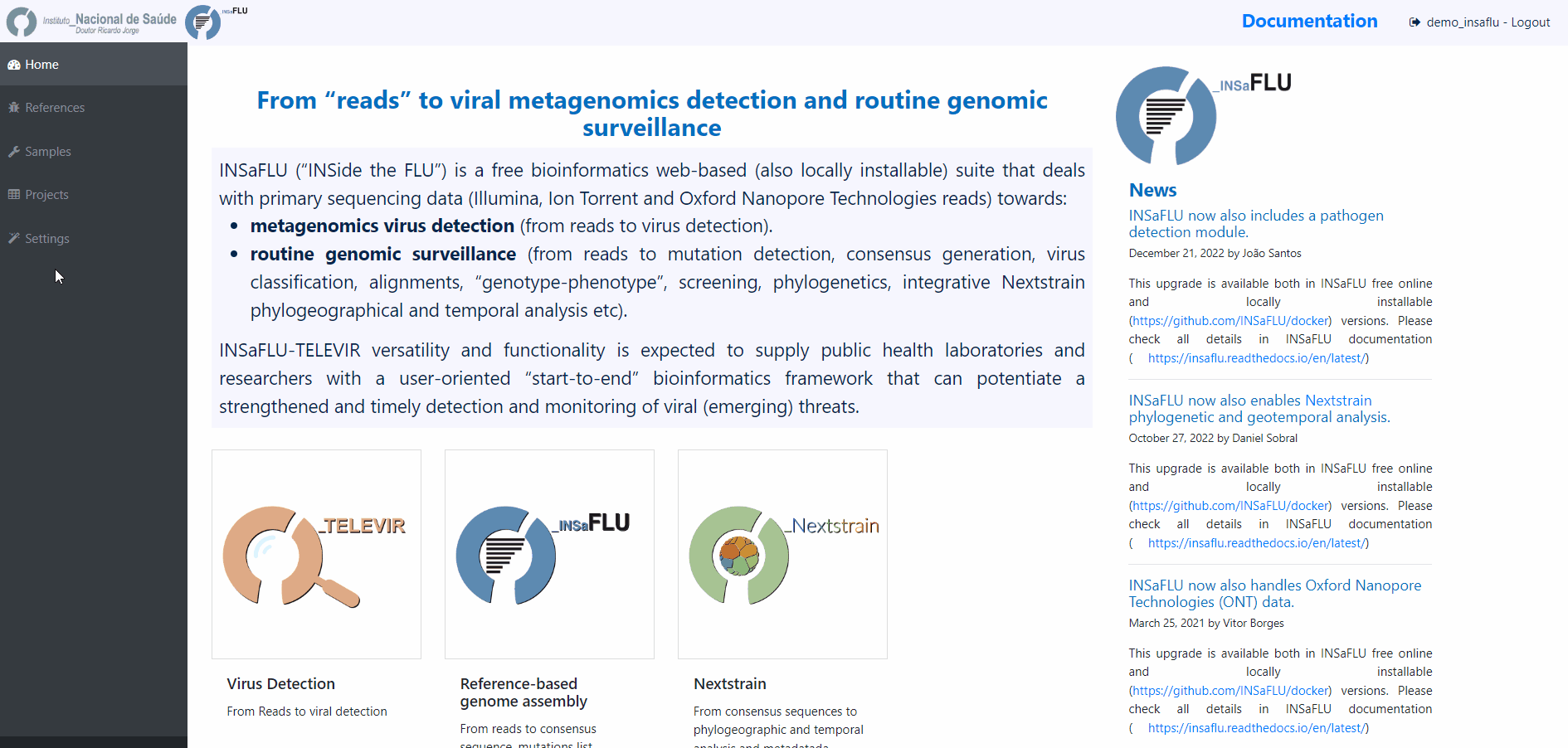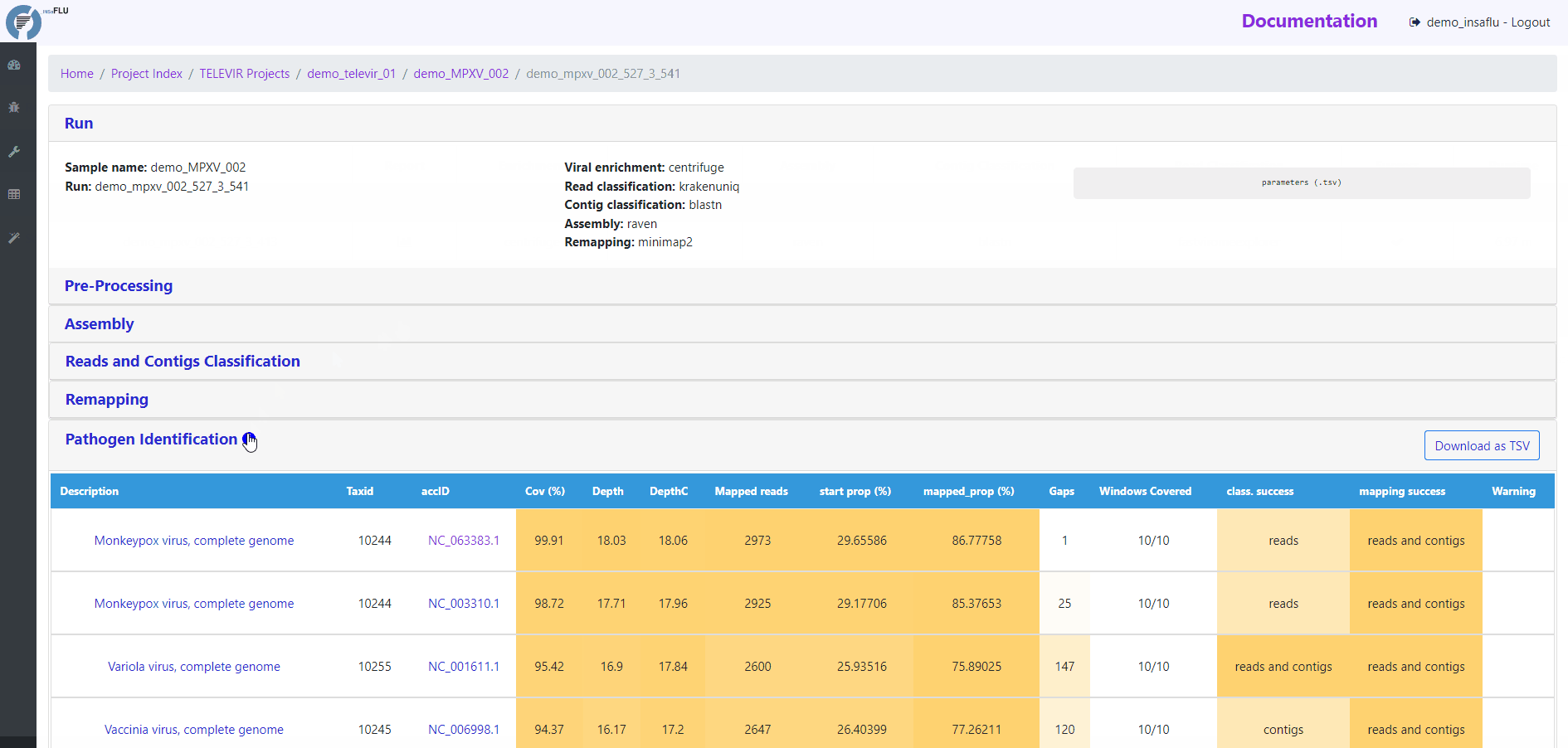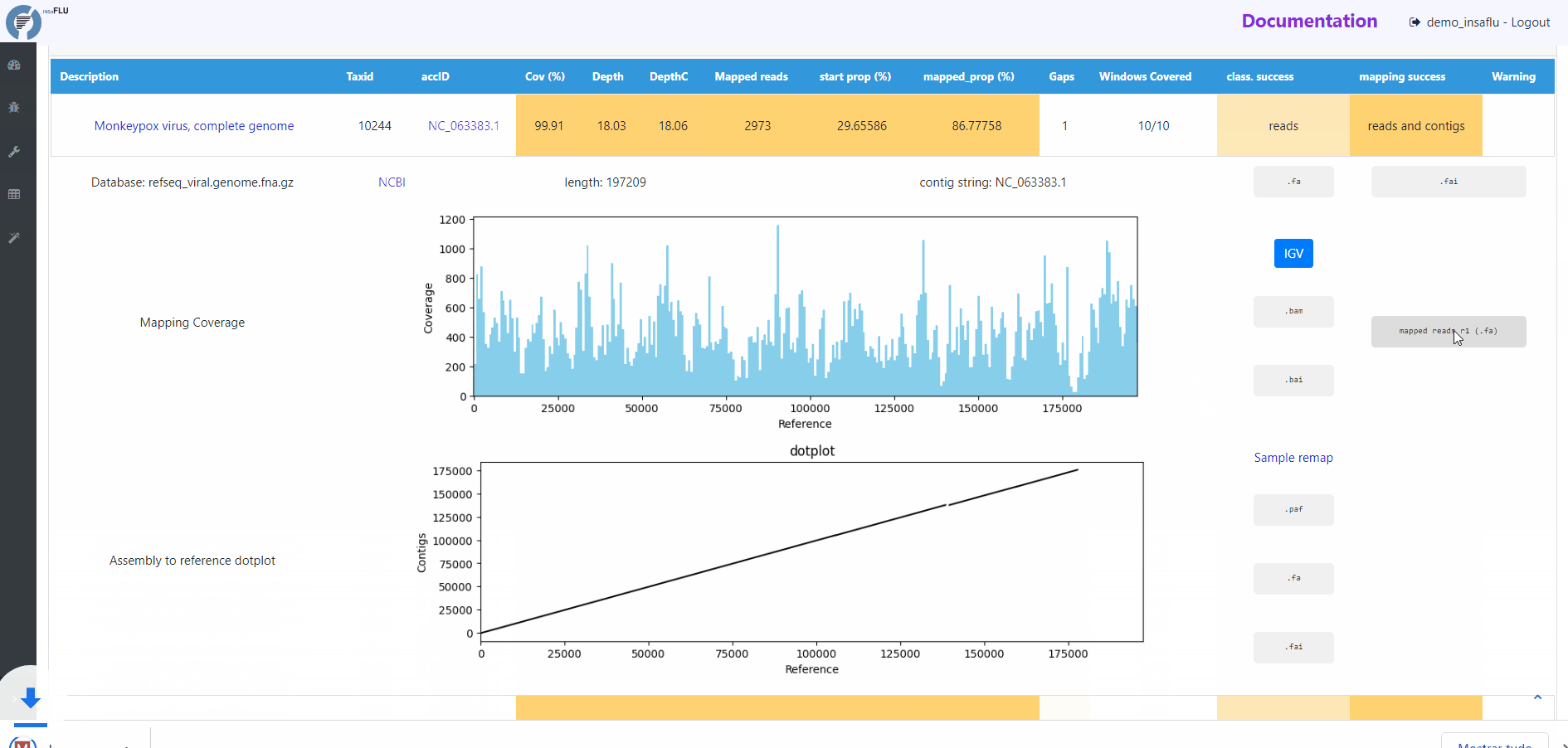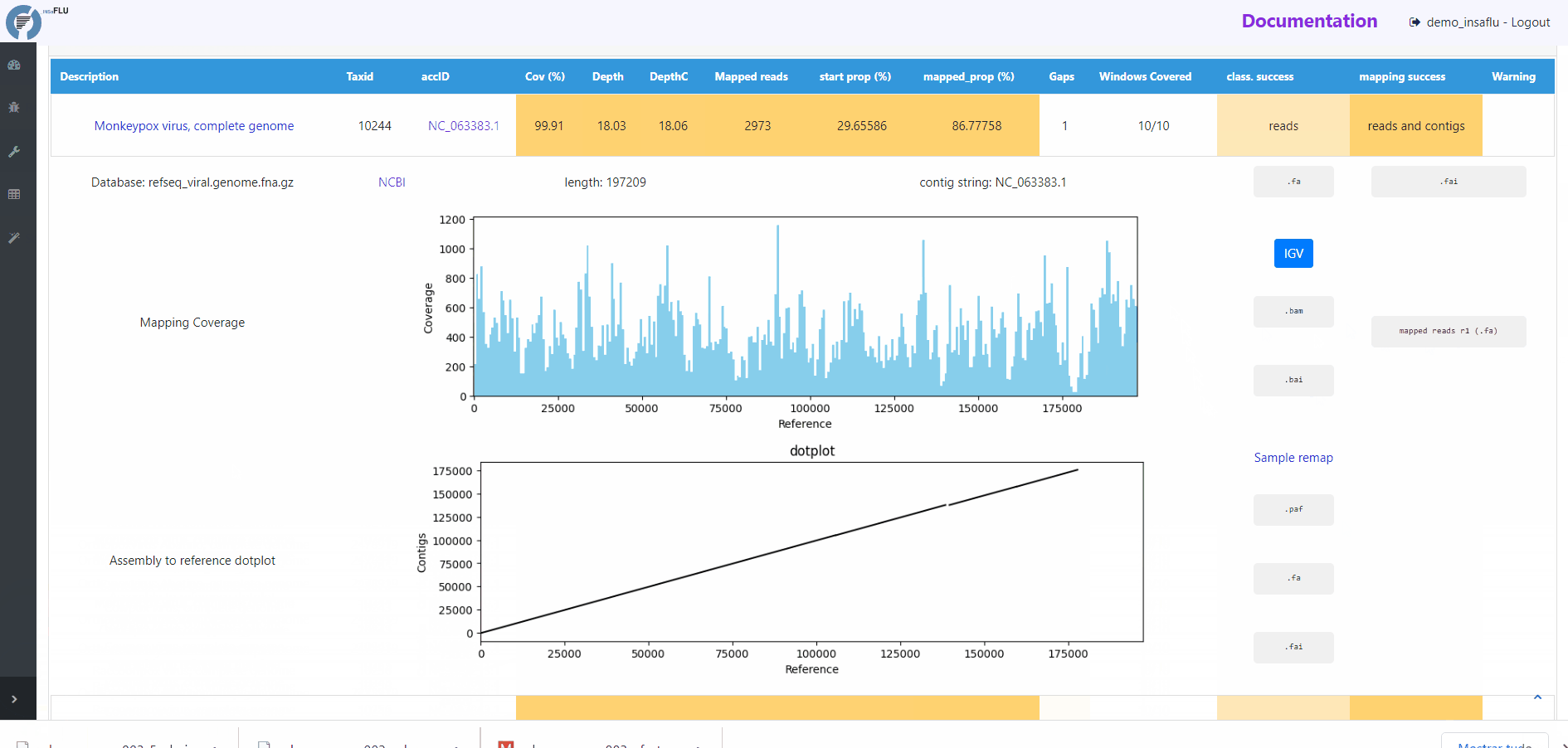
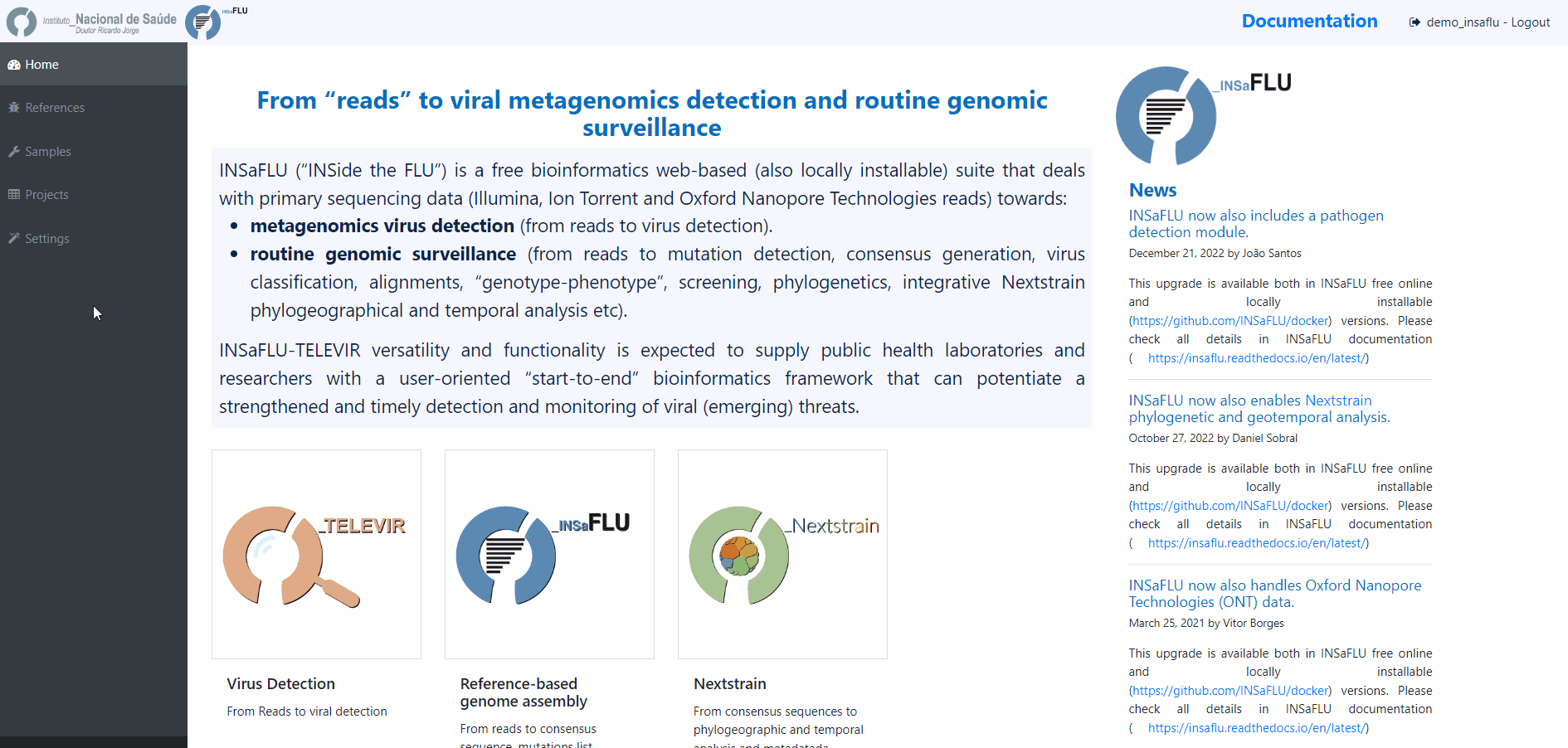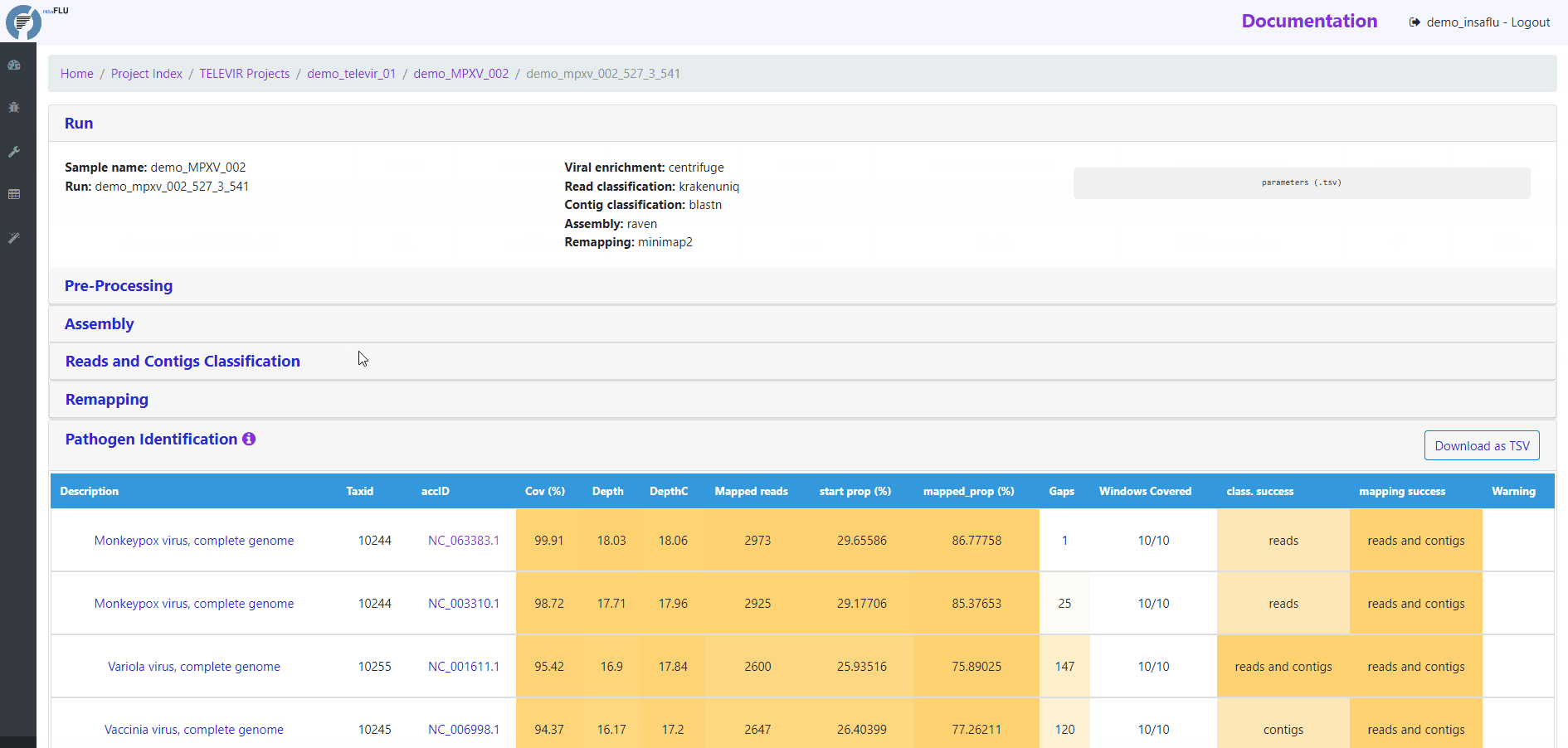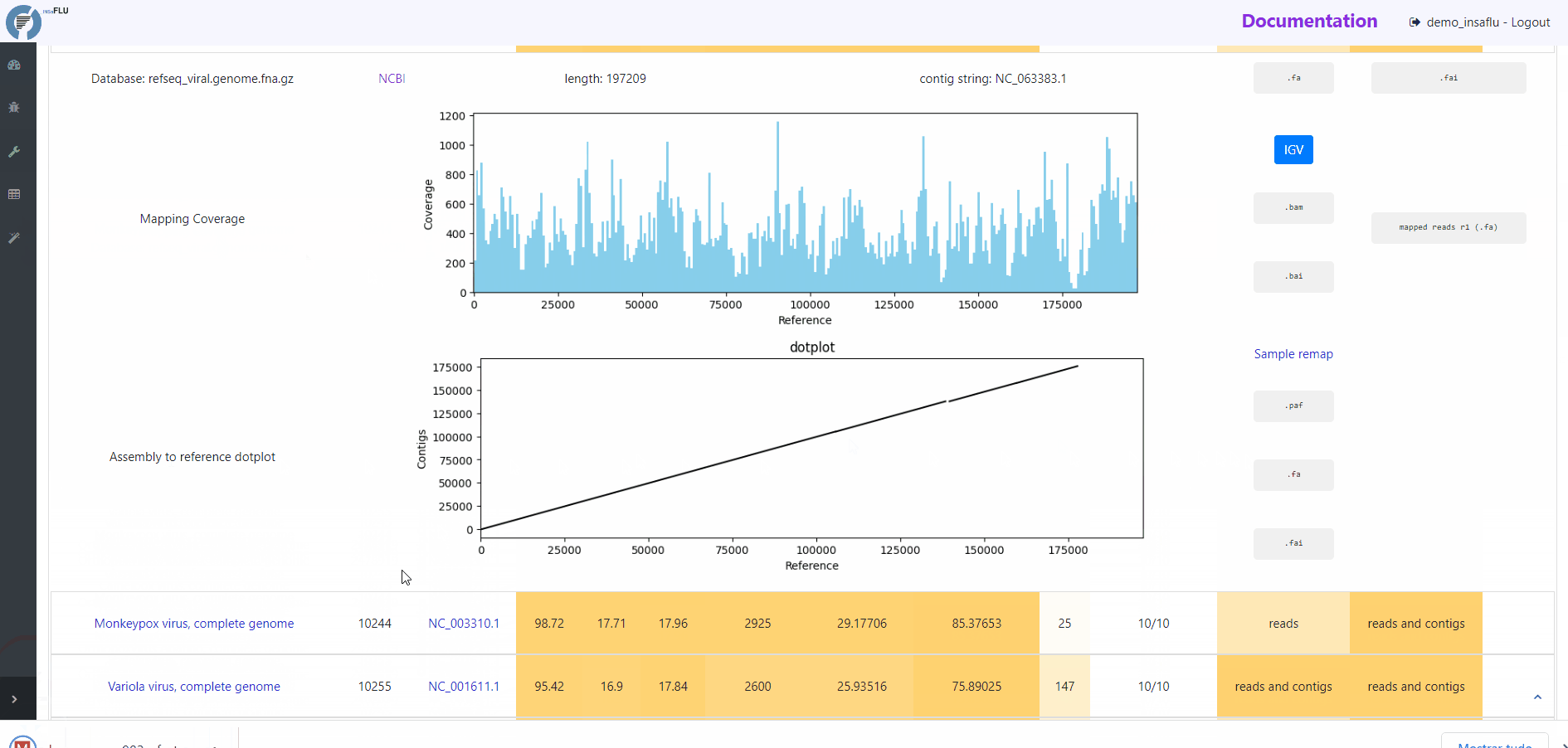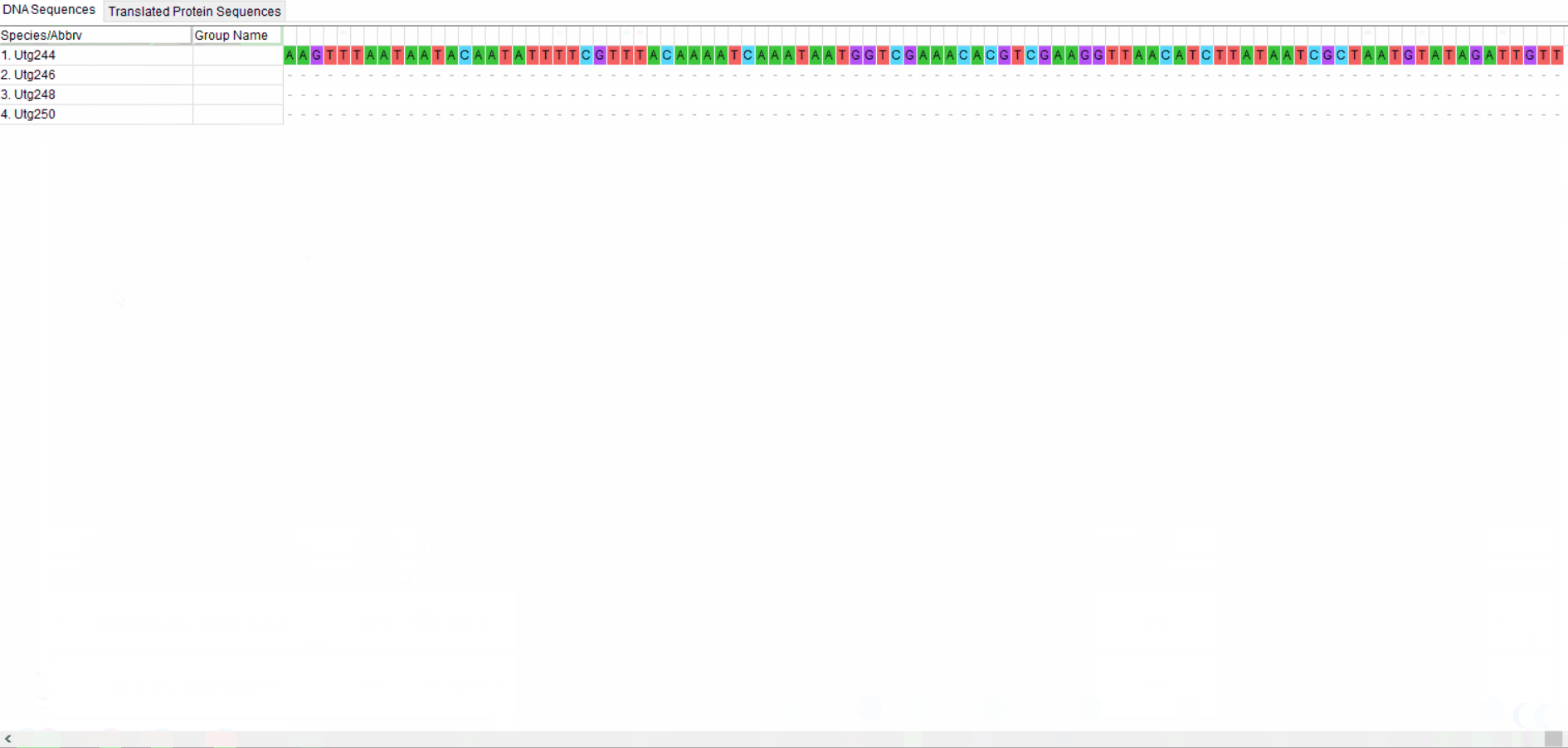
Pathogen identification (Main report)
This tab displays an interactive table with summary statistics and visualizations of the end-point results of the TELEVIR metagenomic virus detection pipeline. In summary, through this pipeline, reads and contigs (if available) are classified independently, then viral hits (TAXID) detected in both intermediate classification reports (reads and contigs) and/or within the top list from each side are selected for reference-based mapping against viral genome sequences present in the available databases. This main report (interactive table) only includes viral hits that were classified at reads and/or contig (“class. success”) level AND that had mapped reads or contigs (“mapping success)
Note
Other viral TAXIDs that were not automatically selected for confirmatory re-mapping step (flagged as “Unmapped”) can be user-selected for mapping at any time by clicking in the “eye” icon available in the Raw Classification and Mapping Summary panel.
Note
The Sample reports have the same layout as this Workflow main report, but compile all viral hits identified accross all workflows that were run a given sample, in which redundant hits are excluded. In summary, one mapping is selected by ACCID. For duplicate refs (the same ACCID) identified in different workflows, the one resulting in higher Cov (%) is presented
The Project reports are simple tables combining all top viral hits identified in the main reports of the several workflows that were run for all samples included in the project.
Both Sample and Project reports provide direct links to the detailed Workflow reports for an enhanced and advanced output interpretation
Important
Viral hits (reference accession IDs) in the main reports (at both “Workflow” and “Sample” levels) can be grouped and sorted by the degree of overlap of cross-mapped reads. This grouping intends to place together true positive hits with their corresponding cross-mapped potential false positives, allowing for the easy identification of the latter. It can be also useful to join same-segment references (for segmented virus) and to help identifying reference sequences most closely related to the virus present in the sample. The grouping parameter (–r-overlap) is modifiable in a new “Reporting” section of the TELEVIR Settings Menu for both technologies. “Sort sample report” should be deployed everytime the grouping parameter is changed for existing projects.
Below, you can find a description of the main outputs and statistics.
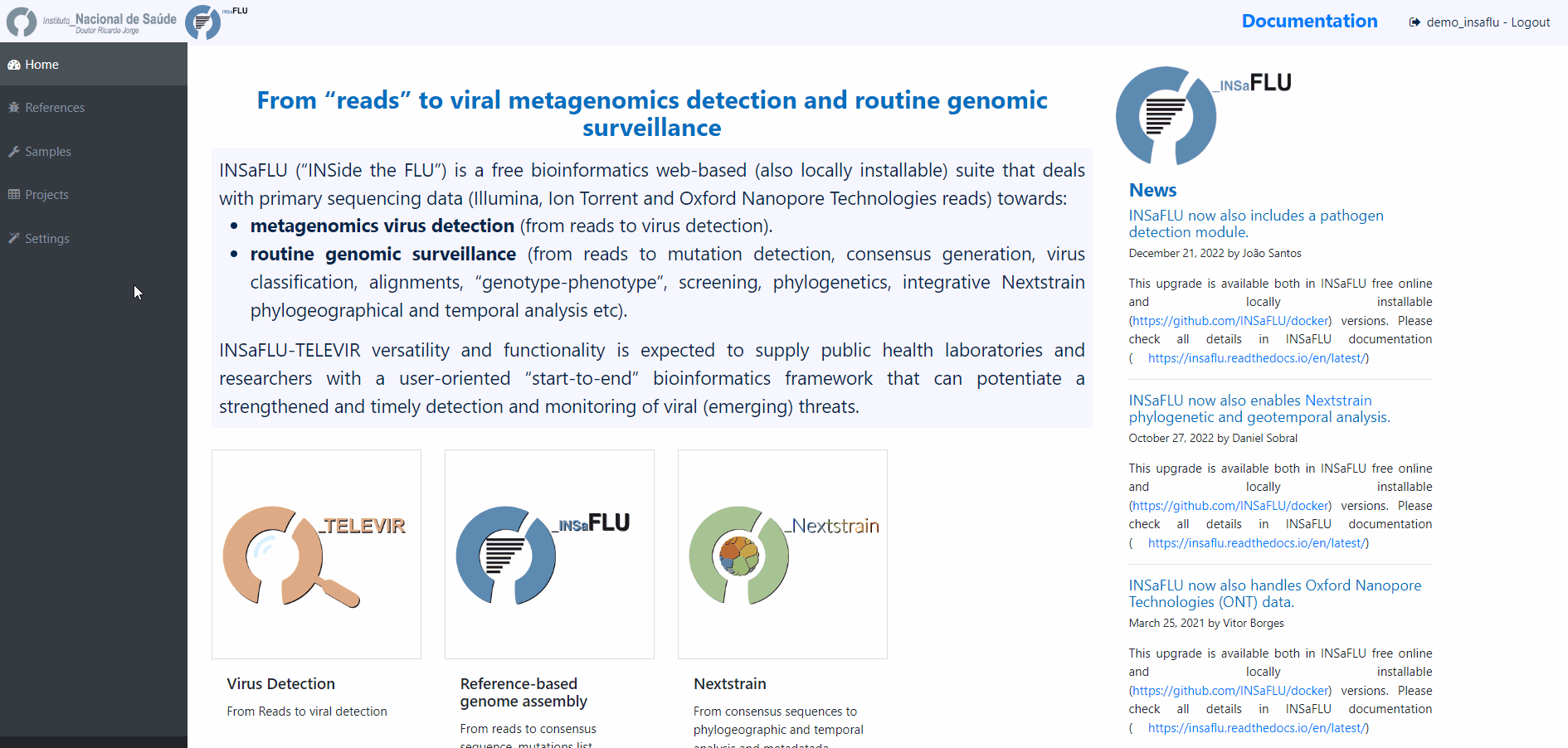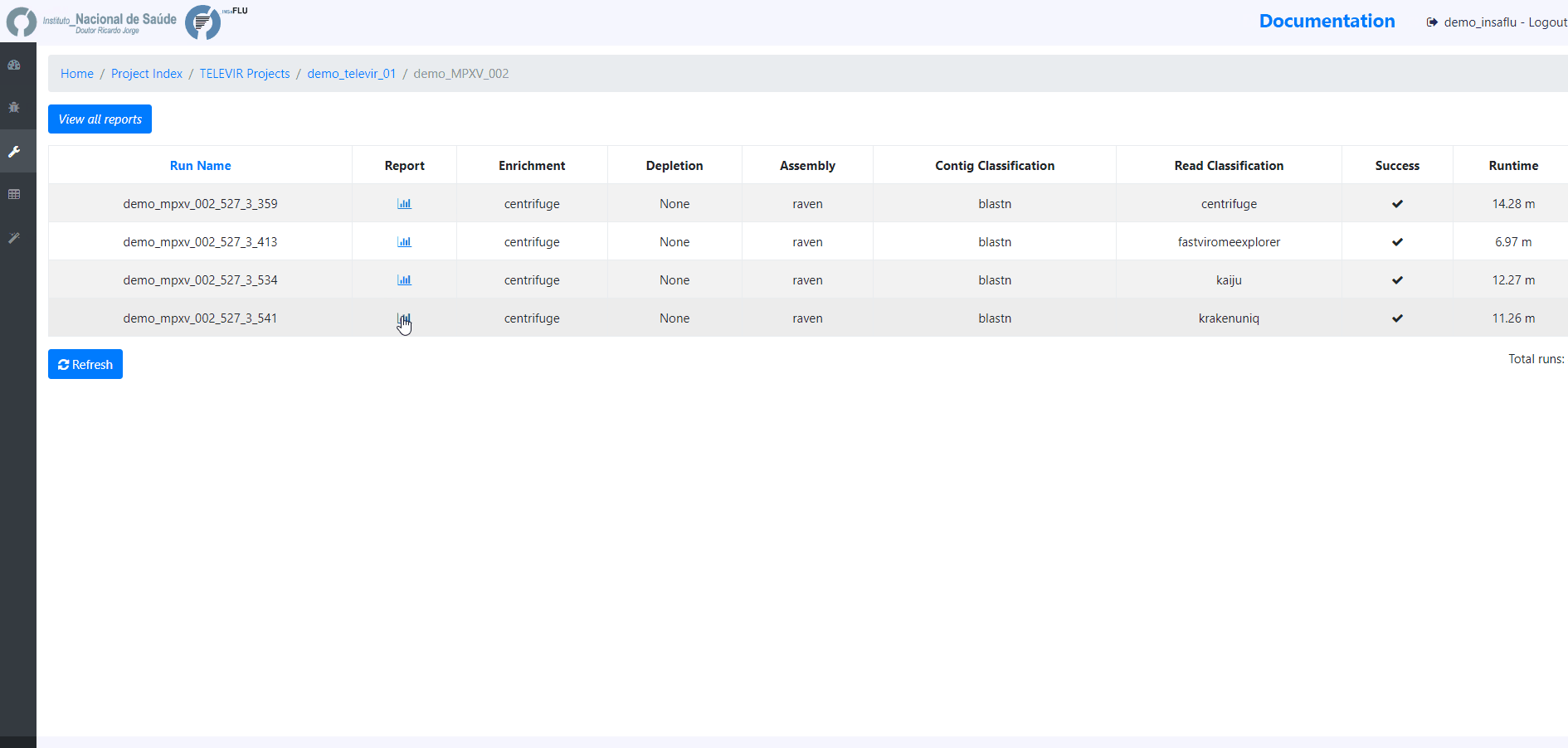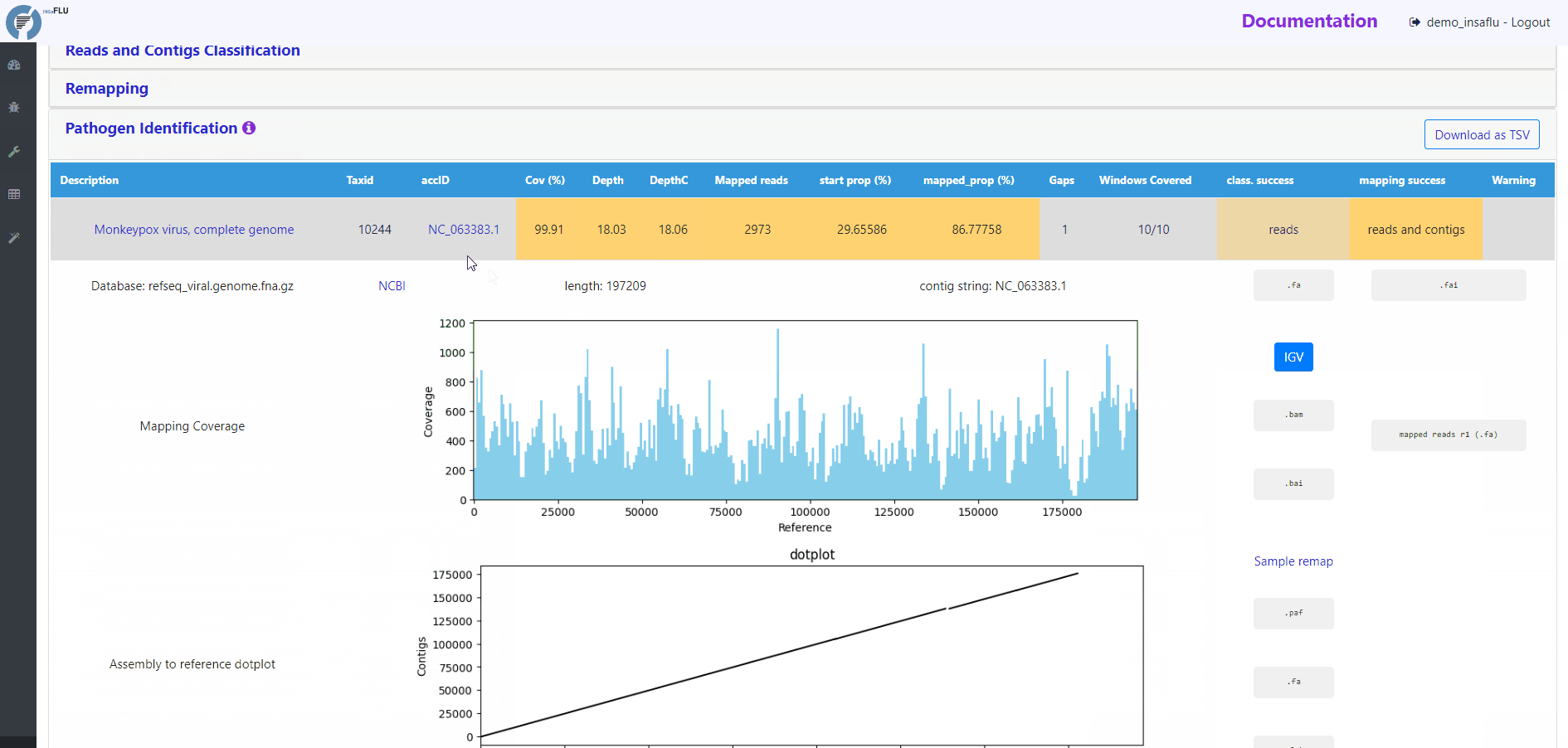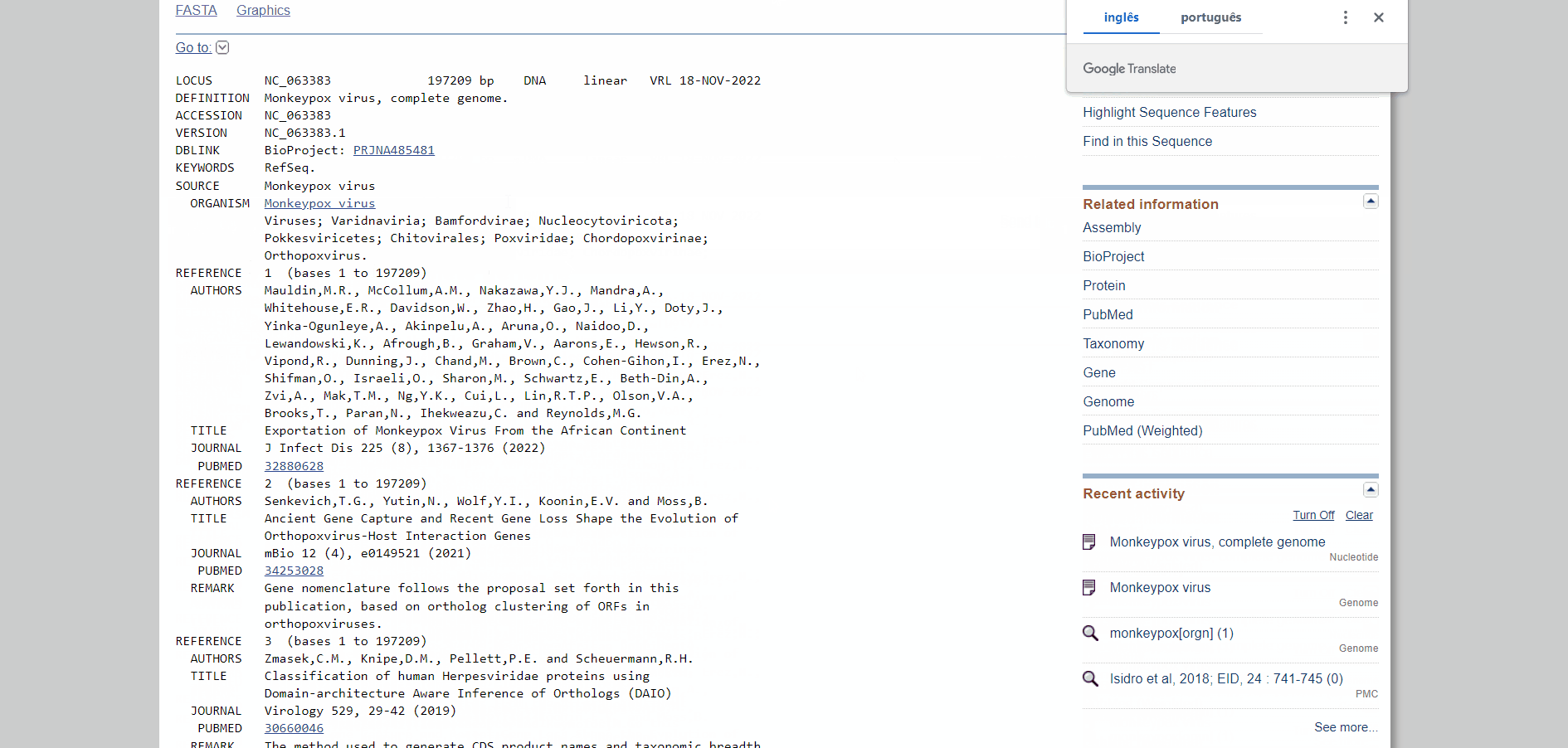
Mapping statistics
Cov (%): horizontal coverage (i.e., percentage of the reference sequence covered)
Depth: mean depth of coverage throughout the whole genome
DepthC: mean depth of coverage exclusively in the covered regions
Mapped reads: number of mapped reads
start prop (%): number of mapped mapped reads divided by the number of input reads (after QC)
mapped_prop (%): number of mapped reads divided by the number of reads used for mapping (i.e., reads retained after the “Virus enrichment” and/or “host depletion steps)
Gaps: number of regions below the minimum coverage threshold (see note below)
Windows Covered: proportion of windows with mapped reads. Reference sequences are split into windows (x), with window size and number (x) being a function of sequence length, from a minimum of 3 up to a maximum of 10. Window number (x) is calculated as the equal division of sequence length by 2000 (without remainder), i.e., sequences <8KB and >20KB result in 3 and 10 windows, respectively.
class. success: indication of whether the TAXID was selected for mapping after reads and/or contigs classification
mapping success: indication of whether reads/and contigs successfully mapped against the TAXID representative references sequence
Warnings:
Flag-type “viruses” (oriented to shotgun metagenomics) (default)
“Likely False Positive”: when most reads map to a very small region of the reference sequence, i.e., hits with high “DepthC” but low “Depth” and low “Cov (%)”. Flagged for hits with DepthC / Depth > 10 and Cov (%) < 5%.
“Vestigial Mapping”: when only a vestigial amount of reads (<= 2) mapped.
Flag-type “probes” (oriented to probe-based NGS target panels)
“Likely False Positive”: when the reference genome is not sufficiently covered as a function of the number of the proportion of Windows Covered, calculated as above. Flagged for hits with Windows Covered <= 50 % (calculated from the fraction presented)
“Vestigial Mapping”: when only a vestigial amount of reads (<= 2) mapped.
Note
Cov is considered only above a minimum Depth threshold. By default, this threshold is set to 1 for ONT data, and to 2 for Illumina data.
For ONT, secondary mappings are suppressed during the re-mapping step. However, supplementary alignments (split or chimeric alignments) are not suppressed , since these can be informative. This behaviour can result in higher coverage than the number of reads mapped.
Mapping plots and output files
By clicking in a TAXID description, user can visualize/download multiple outputs regarding:
# READS MAPPING
Mapping Coverage plot (depth of coverage throughout the reference genome)
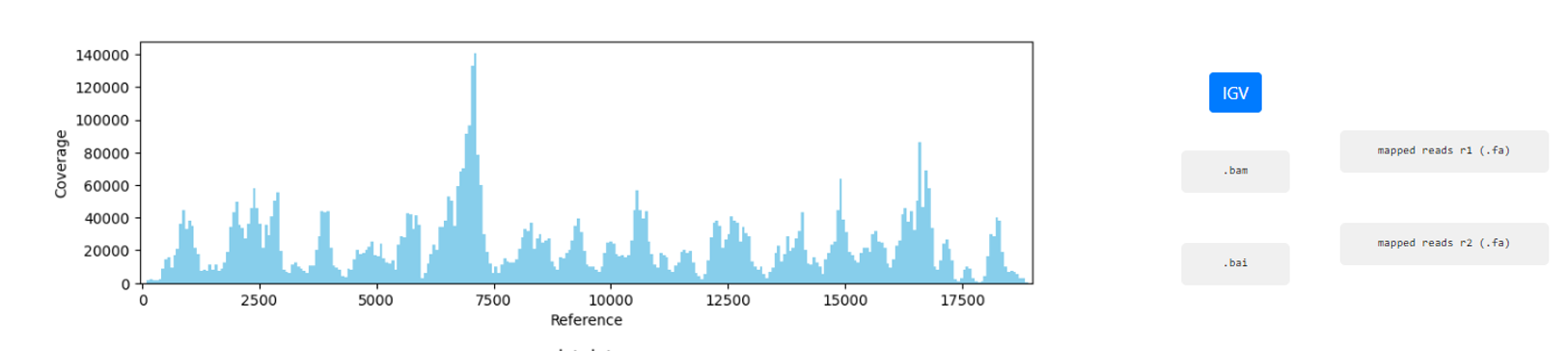
Integrative Genomics Viewer (IGV) visualization of the mapped reads
Mapped reads in FASTA and BAM
Reference sequence (“.fa” format) and “.fai” index
# CONTIGS MAPPING
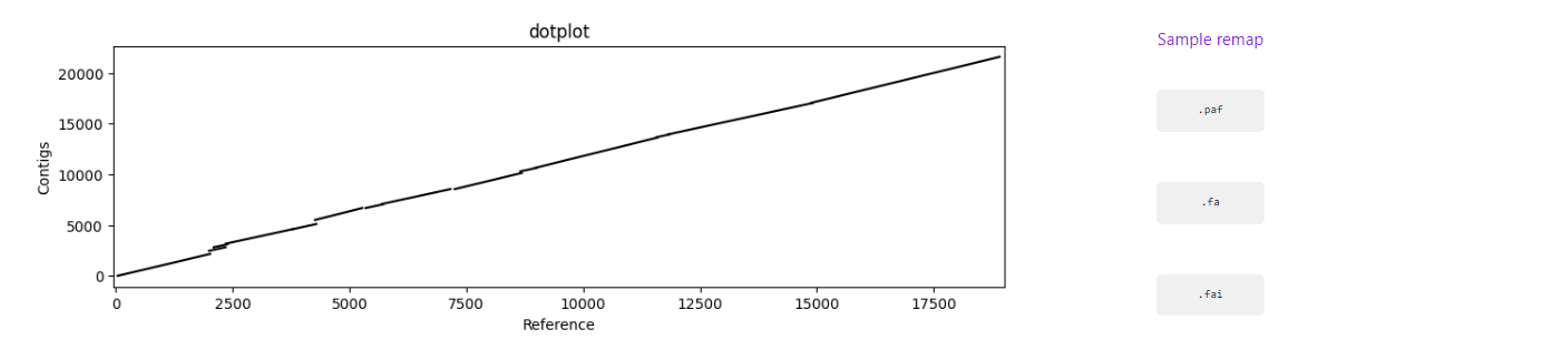
Assembly to reference dotplot (location of the mapped contigs into the reference sequence)
Mapped contigs in FASTA
Contigs alignment in Pairwise mApping Format (PAF)
Sample remap page: statistics regarding the reads’ mapping against the set of contigs classified for a given TAXID.
Guide for report interpretation
Interpretation of metagenomics virus detection data is not a trivial task (even for users with expertise in virology and/or bioinformatics). In order to facilitate output interpretation and decision-making on the part of users, TELEVIR runs culminate in user-oriented reports with a list of the top viral hits, each accompanied by several robust and diagnostic-oriented metrics (described above). Here, we provide some guidance on how to interpret TELEVIR reports and exclude/confirm viral hits, by exemplifying “expected” metric profiles (or combination of profiles) when there are differents levels of evidence for the virus presence:
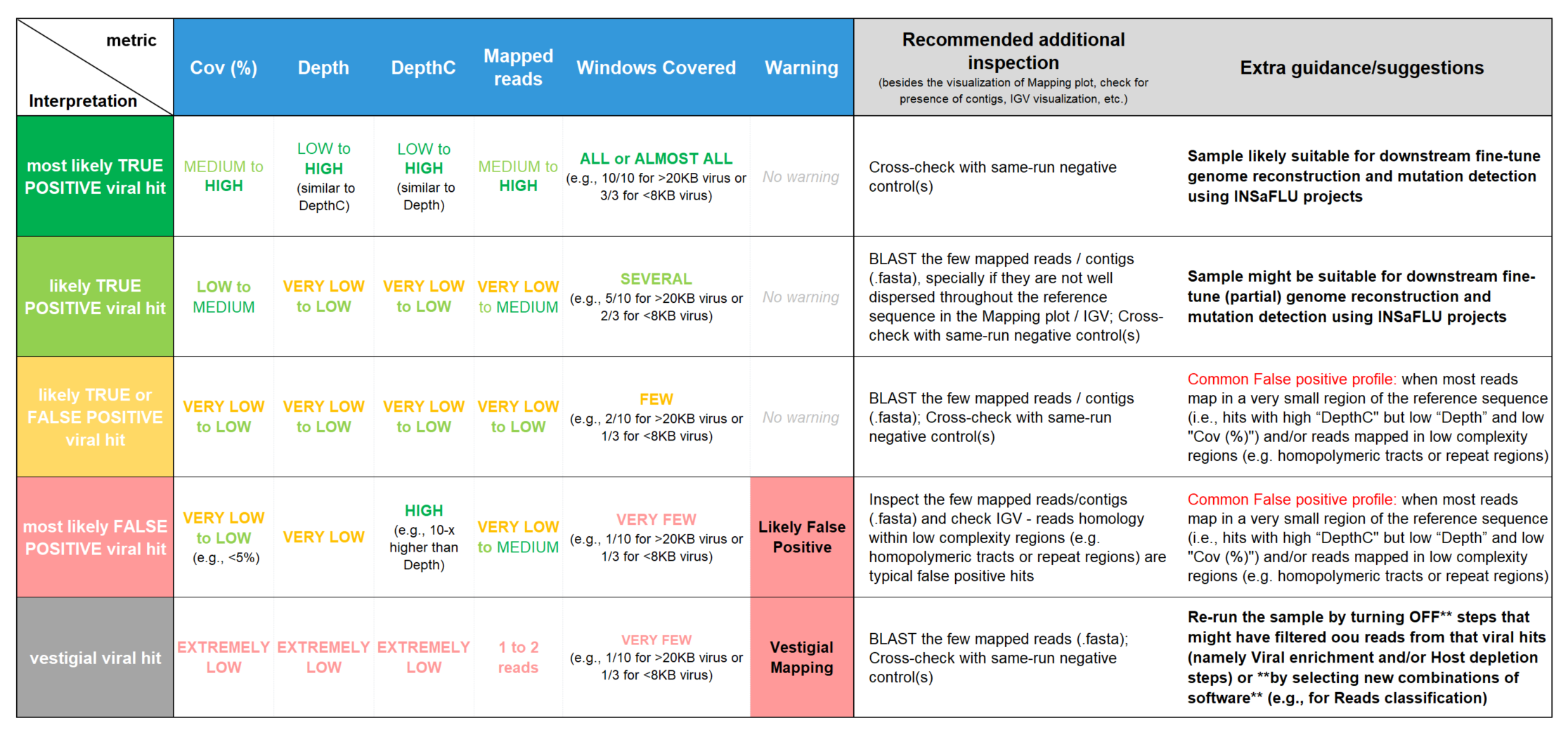
Further guidance:
# Why do you have
MULTIPLE HITS FOR THE SAME VIRUS (TAXID)?. This is likely due to the presence of:
segmented virus(es) in the sample (each reference segment has different accession numbers, so they are listed in different rows). In this case, if segmented and non-segmented viruses are expected to be present in the sample, it might worth checking the Raw Classification table and requesting extra mapping (as the top hits listed in the Main report might have not included the non-segmented virus due to the over listing of the segmented ones).
several reference genomes (strains/variants) of the same virus in the available Viral reference databases. In this case, the virus present in the sample is likely more closely related to the reference genome (accession number) yielding the best mapping metrics.
MULTIPLE HITS FOR CLOSELY RELATED TAXID? This is likely due to the cross-mapping of reads across several reference genomes with considerable nucleotide homology, such as viruses belonging to the same family. In this case, the virus present in the sample is likely more closely related to the reference virus (TAXID) yielding the best mapping metrics. INSaFLU team is working to facilitate grouping hits by virus genetic relatedness…
# What should you do if your expected virus is not listed in the Main report?
Check if the expected virus is listed in the Raw Classification and Mapping Summary panel. If it is listed and is flagged as “Unmapped”, it means that the virus is likely present at a very low amount in the sample (and, as such, it was not automatically selected for confirmatory re-mapping step). Click in the “eye” icon to request confirmatory mapping. The results will show up soon in the Main table report.
Re-run the sample by turning OFF steps that might have filtered out your expected virus (namely Viral enrichment and/or Host depletion steps) or by selecting new combinations of software (e.g., for Reads classification).
Despite INSaFLU-TELEVIR platform is taking advantage of several viral reference databases, they do not cover all viruses. For instance, newly discovered or uncommon virus or viral strains (e.g., viruses without available complete genomes) might be missing, leading to false negative results.
The ultimate goal of the TELEVIR module is to detect viruses, and not necessarily to identify the virus “strain/variant/serotype”. Once a given virus is detected, users can perform fine-tune analyses (e.g, consensus sequences reconstruction, mutation detection, etc) with the classical INSaFLU projects.
# How can you compare your test samples with the “negative/positive controls”?
The inclusion of negative controls (e.g. pathogen-negative samples, library preparation buffers, etc) during metagenomic sequencing in clinical virology is highly recommended to identify sources of potential contamination and detect false positive hits. Indeed, viral taxa/sequences detected in the test samples that are also present in the negative run controls should be interpreted as contamination (e.g., during wet-lab steps) or background noise (e.g., nucleic acids present in wet-lab reagents might yield false positive viral hits across test and control samples). In another perspective, the inclusion of positive controls (e.g., samples spiked with RNA or DNA viruses that do not infect humans) is also recoomended to control for the success of nucleic acids extraction, preparation and sequencing.
In this context, INSaFLU-TELEVIR users are encouraged to create different TELEVIR projects per different metagenomics sequencing run (including negative/positive controls) for an enhanced sample comparison and output interpretation.
After selection of “control” sample(s) (which can be done before and after data analysis), viral TAXID detected in the Main report of the user-selected “control” sample(s) will be flagged in the reports of the other samples as “Taxid found in control” in the “Control” column. This new functionality is designed to facilitate the background subtraction of viral hits also found in controls. Multiple controls are possible.
For further recommendations for interpretation of metagenomics virus detection data, we recommend the following literature:
de Vries JJC, et al, 2021. Recommendations for the introduction of metagenomic next-generation sequencing in clinical virology, part II: bioinformatic analysis and reporting. J Clin Virol. https://doi.org/10.1016/j.jcv.2021.104812
López-Labrador FX et al, 2020. Recommendations for the introduction of metagenomic high-throughput sequencing in clinical virology, part I: Wet lab procedure. J Clin Virol. https://doi.org/10.1016/j.jcv.2020.104691
Intermediate outputs
Multiple intermediate outputs and statistics are available by clicking in the following ‘expand-and-collapse’ panels:
Assembly
This tab provides an overview on the assembly step (thi steps uses the reads retained after the “Viral enrichment” and/or “Host depletion” steps).
Filtered contigs are provided for download (fasta.gz format).
Reads and Contigs classification
This tab provides reads and/or contigs classification reports (tsv format) with the list of viral hits (TAXID and representative accession numbers) detected after the intermediate screening against viral sequence databases. The two reports are merged to select the top viral hits to be automatically subjected to confirmatory re-mapping (see next steps). These reports are also compiled in the Raw Classification and Mapping Summary panel (below).
Raw Classification and Mapping Summary
This table lists all viral hits (TAXID and representative accession numbers) detected during the intermediate step of Reads and Contigs Classification (see above), indicating if they were (or not) automatically selected for confirmatory re-mapping.
TAXIDs that were not automatically selected for confirmatory re-mapping step (flagged as “Unmapped”) can be user-selected for mapping at any time by clicking in the “eye” icon. The result of the user-requested mapping will show up in the Main table report.